The structure of isobutane is _________
Video Solution
Text Solution
NA
|
Similar Questions
Knowledge Check
The IUPAC name of isobutane is ______.
A2-MethylbutaneBButaneC2- MethylpropaneD2,2-DimethylpropaneThe percentage of 1-bromo-2-methyl propane obtained in the photochlorination of isobutane is about
A0.46B0.64C0.56D0.01The structure of D-(+) glucose is

The structure of L-(-) glucose is
 AStructure (i)BStructure (ii)CBoth (i) & (ii)DNeither (i) nor (ii)
AStructure (i)BStructure (ii)CBoth (i) & (ii)DNeither (i) nor (ii)
A cooking gas cylinder is assumed to contain 11.2kg isobutane. The combustion of isobutane is given by :
C4H10(g)+(13/2)O2(g)→4CO2(g)+5H2O(l),ΔH=−2658kJmol−1 (a) If a family needs 15000kJ of energy per day for cooking, how long would the cylinder last ?
(b) Assuming that 30% of the gas is wasted due to incomplete combustion, how long would the cylinder last ?
View SolutionThe structure of D-(+) glucose is

the structure of L-(-) glucose is
View SolutionThe structure of D−(+)− glucose is

The structure of L−(−)− glucose is
View SolutionThe structure of D−(+)− glucose is
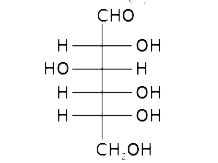
The structure of L−(−) glucose is
View SolutionIdentify which of the structures below are meso structures ?
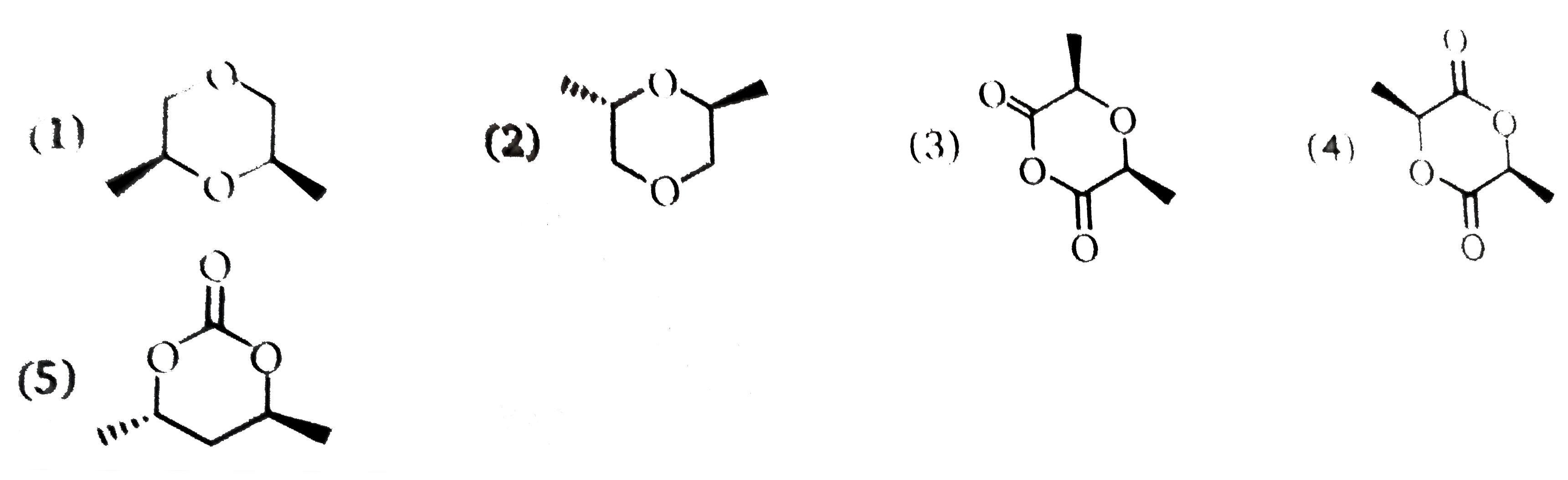
View Solution
