Describe the laboratory method of preparation of hydrogen gas.
Video Solution
Text Solution
Laboratory method of preparation
Principle: All the metals present above hydrogen in the reactivity series on reaction with dilute HCI or dilute H2SO4 liberate hydrogen gas. Zinc metal is preferred for this reaction.
Zn+2HCI→ZnCI2+H2↑
Zn+H2SO4→ZnSO4+H4↑
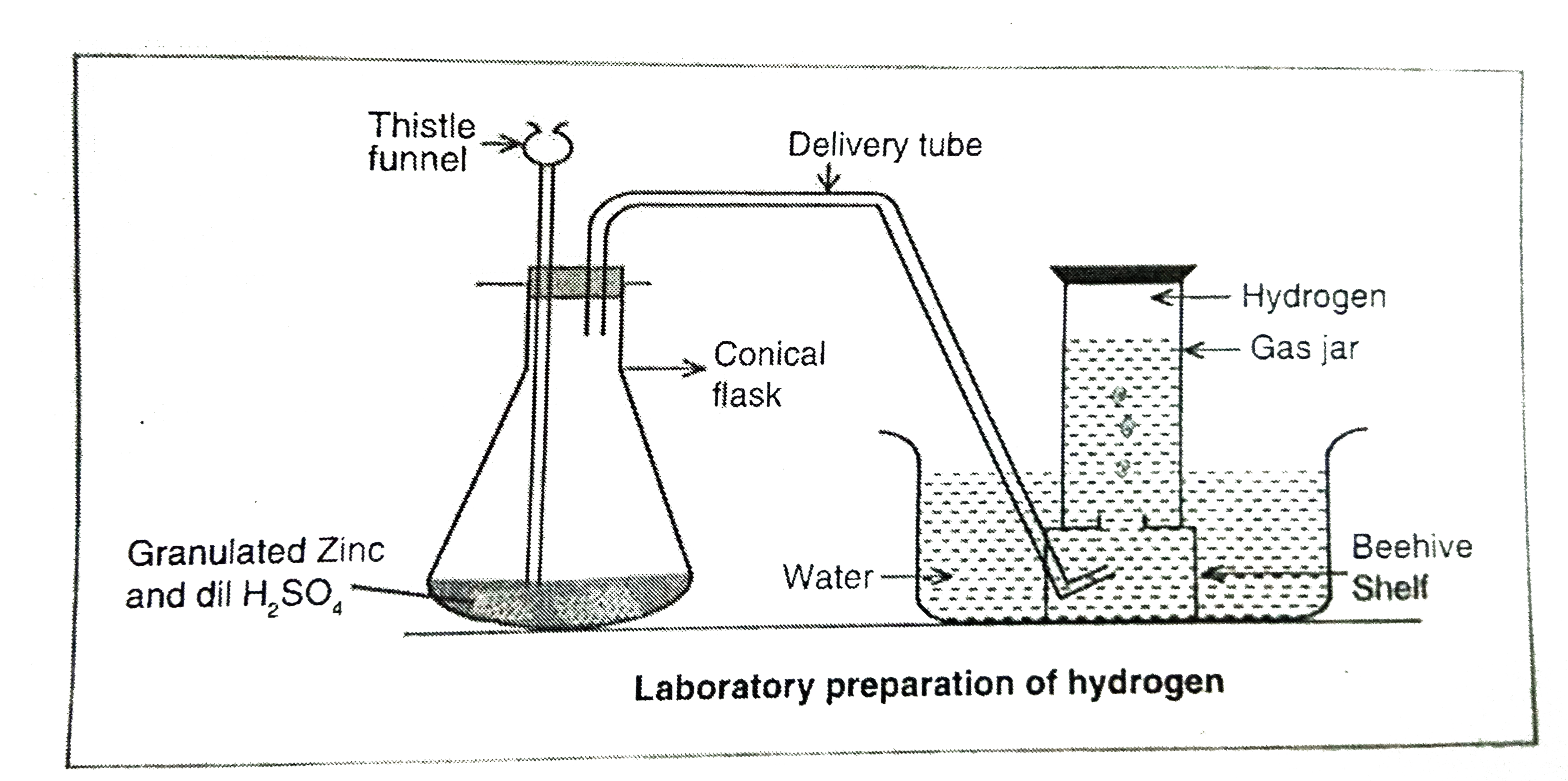
Experiment : Some granulated zinc pieces are taken in a flat-bottom flask bottom flask fitted with a two holed air tigth cork. In one hole, a thistle a thistle funnel is inerted and in anoter hole, a long dilivery tube is inseted. Dilute H2SO4 is added to the flask drop-wise through the thistile funnel.
Observation: Effervescence is observed in the initial stages of the reaction. Then a colourless, and odourless gas evolves.
Collection of gas: Hydrogen is collected by the downward displacement of water.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
The laboratory method of preparation of CO is
AHCOOHH2SO4−−−→H2O+COBCO2+C→2COCH2CO3Heat−−→H2O+CO+12O2D2C+O2→2COIn the laboratory method of preparation of dioxygen by thermal decomposition of potassium chlorate the catalyst used is
AIron fillingsBManganese dioxideCPlatinumDCopper chlorideLaboratory method for the preparation of acetyl chloride is :
ACH3COOH+SOCl2→CH3COClBCH3COOH+PCl3→CH3COClCCH3COONa+PCl3→CH3COClDAll of these
Describe a laboratory method of the preparation of wood charcoal.
View SolutionDescribe laboratory method for preparation of glucose . Write the reaction that indicates the presence of - CHO group in glucose .
View SolutionDescribe the laboratory preparation of oxygen gas from hydrogen peroxide with a neat diagrem.
View Solution(a) Write the laboratory method for the preparation of HNO3.
(b) Write the structure of three oxoacids of phosphorus.
View SolutionGive reason for the following:
Zinc granules are preferred for the laboratory preparation of hydrogen rather than pure zinc.
View Solution
