Nitrates on thermal decomposition give oxygen.
Step-by-Step Solution:
1. Understanding Thermal Decomposition:
- Thermal decomposition is a chemical reaction where a compound breaks down into simpler substances when heated. In this case, we are focusing on nitrates.
2. Identifying the Example:
- We will use potassium nitrate (KNO3) as an example to illustrate the thermal decomposition of nitrates.
3. Writing the Decomposition Reaction:
- When potassium nitrate is heated, it decomposes into potassium nitrite (KNO2) and oxygen (O2). The balanced chemical equation for this reaction is:
2KNO3(s)→2KNO2(s)+O2(g)
4. Conclusion:
- From the above reaction, we can conclude that nitrates, when subjected to thermal decomposition, release oxygen gas as one of the products.
- Thermal decomposition is a chemical reaction where a compound breaks down into simpler substances when heated. In this case, we are focusing on nitrates.
- We will use potassium nitrate (KNO3) as an example to illustrate the thermal decomposition of nitrates.
- When potassium nitrate is heated, it decomposes into potassium nitrite (KNO2) and oxygen (O2). The balanced chemical equation for this reaction is:
2KNO3(s)→2KNO2(s)+O2(g)
- From the above reaction, we can conclude that nitrates, when subjected to thermal decomposition, release oxygen gas as one of the products.
Topper's Solved these Questions
- AIR AND OXYGENBook:PEARSON IIT JEE FOUNDATIONChapter:AIR AND OXYGENExercise:LEVEL-2
Explore 25 Videos
- AIR AND OXYGENBook:PEARSON IIT JEE FOUNDATIONChapter:AIR AND OXYGENExercise:LEVEL-3
Explore 10 Videos
- AIR AND OXYGENBook:PEARSON IIT JEE FOUNDATIONChapter:AIR AND OXYGENExercise:ESSAY TYPE QUESTIONS
Explore 5 Videos
- ATOMIC STRUCTUREBook:PEARSON IIT JEE FOUNDATIONChapter:ATOMIC STRUCTUREExercise:Level 3
Explore 10 Videos
Similar Questions
Knowledge Check
Potassium nitrate on thermal decomposition gives
AN2BO2CH2DO3Thermal decomposition of
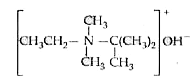 givesACH3CH=CH2BCH2=CH2CCH3−CH3DCH3CH2CH3
givesACH3CH=CH2BCH2=CH2CCH3−CH3DCH3CH2CH3Which of the following on thermal decompositrion gives oxygen gas ?
AAg2OBPb3O4CPbO2DAll of these
Give two examples of thermal decomposition.
View SolutionThe correct order of ease of thermal decomposition is -
View SolutionWhat is meant by thermal decomposition ? Give an example.
View SolutionNitrogen gas is liberated by thermal decomposition of:
View SolutionThe compound that does not produce nitrogen gas upon thermal decomposition is :
View Solution