Why does boiling of water expel the dissolved gases ?
Text Solution
As temperature increases the solubility of a gas in a liquid decreases. Hence boiling of water expels he dissolved gases.
|
Similar Questions
Knowledge Check
Which of the following types of water does not contain dissolved gases such as oxygen and carbon dioxide?
APotable waterBDistilled waterCSaline waterDSoft waterWhich of the following types of water does not contain dissolved gases such as oxygen and carbon dioxide?
APotable waterBDistilled waterCSaline waterDSoft waterWhich of the following types of water does not contain dissolved gases such as oxygen and carbon dioxide?
Apotable waterBdistilled waterCsaline waterDsoft water
Why does a salt disappear when dissolved in water ?
View SolutionWhy does sound travel faster in solids that in gases?
View SolutionThe given below experiment is to show tap water contains dissolved gases. Based on this, answer the below questions:
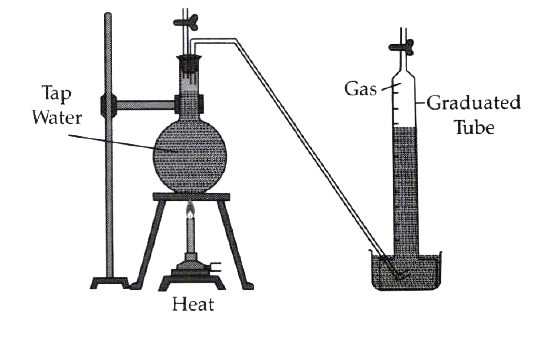
How are the gases dissolved in water can be easily expelled?
View SolutionWhich of the following types of water does not contain dissolved gases such as oxygen and carbon dioxide?
View SolutionWhich among the following is not an application of dissolved gases in water?
View Solution
