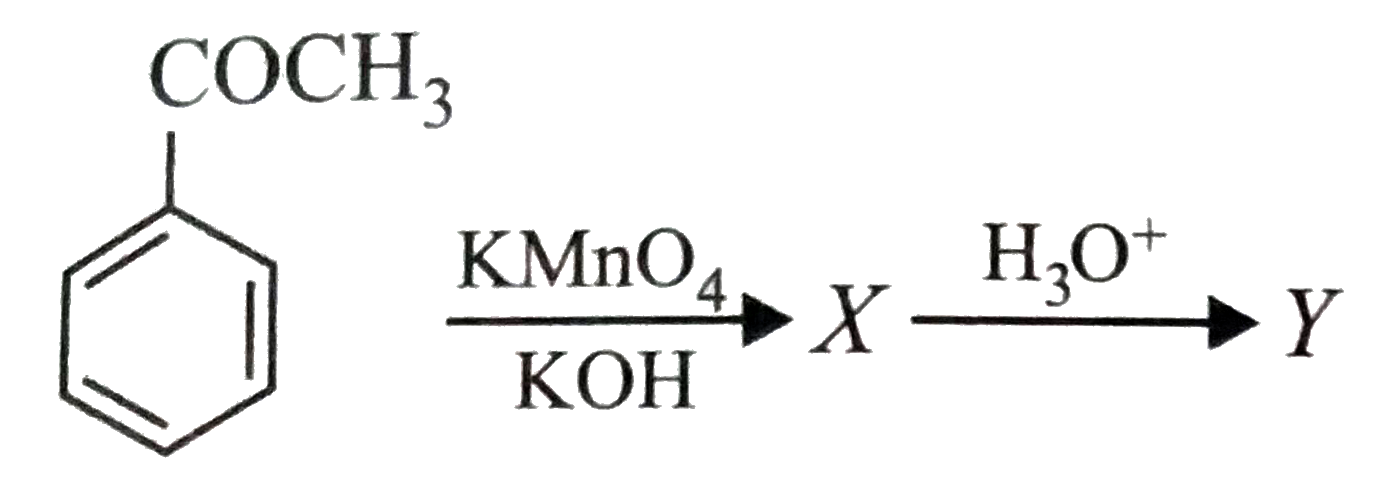Two substances X and Y are dissolved in water under suitable conditions. X is a gas while Y is a solid under normal conditions. Solution of Y is found to conduct electricity but not 'X'. Based on the conclusion identify the nature of bond present in X and Y with appropriate reasons as given below.
Arrange the steps given below in a sequence.
(1) Y is a solid at room temperature and its aqueous solution conducts electricity. This shows that it is an ionic compound.
(2) X on dissolution in water does not conduct electricity and is a gas at room temperature. Hence it should be a non-polar covalent molecule.
(3) Aqueous solutions of both ionic compounds and polar covalent compounds conduct electricity because of the presence of free ions.
(4) All ionic compounds are solids and most of the non-polar covalent molecules are gases or solids at room temperature. Polar covalent compounds are liquids or gases.
A
3 4 2 1
B
1 4 3 2
C
1 3 2 4
D
3 1 2 4
Text Solution
(i) Aqueous solutions of both ionic compounds and polar covalent compounds conduct electricity because of the presence of free ions.
(ii) All the ionic compounds are solids and most of the non-polar covalent molecules are gases or solids at room temperature. Polar covalent compounds are liquids or gases.
(iii) As aqueous solution of X doesn't conduct electricity and moreover it is a gas at roo, temperature. It should be a non-polar molecule. Hence, it is a covalent molecule.
(iv) Y is a solid at room temperature in which its aqueous solution conducts electricity shows that it is an ionic compound.
|
Topper's Solved these Questions
- CHEMICAL BONDING BOOK - PEARSON IIT JEE FOUNDATIONCHAPTER - CHEMICAL BONDING EXERCISE - Level 2
21 Videos
- CHEMICAL BONDING BOOK - PEARSON IIT JEE FOUNDATIONCHAPTER - CHEMICAL BONDING EXERCISE - Level 3
10 Videos
- CHEMICAL BONDING BOOK - PEARSON IIT JEE FOUNDATIONCHAPTER - CHEMICAL BONDING EXERCISE - Essay type questions
2 Videos
- CHEMICAL KINETICS AND EQUILIBRIUMBOOK - PEARSON IIT JEE FOUNDATIONCHAPTER - CHEMICAL KINETICS AND EQUILIBRIUMEXERCISE - Level 3
10 Videos
- ElectrochemistryBOOK - PEARSON IIT JEE FOUNDATIONCHAPTER - ElectrochemistryEXERCISE - Level 3
10 Videos
Similar Questions
Knowledge Check
Identify X and Y in the given sequence.
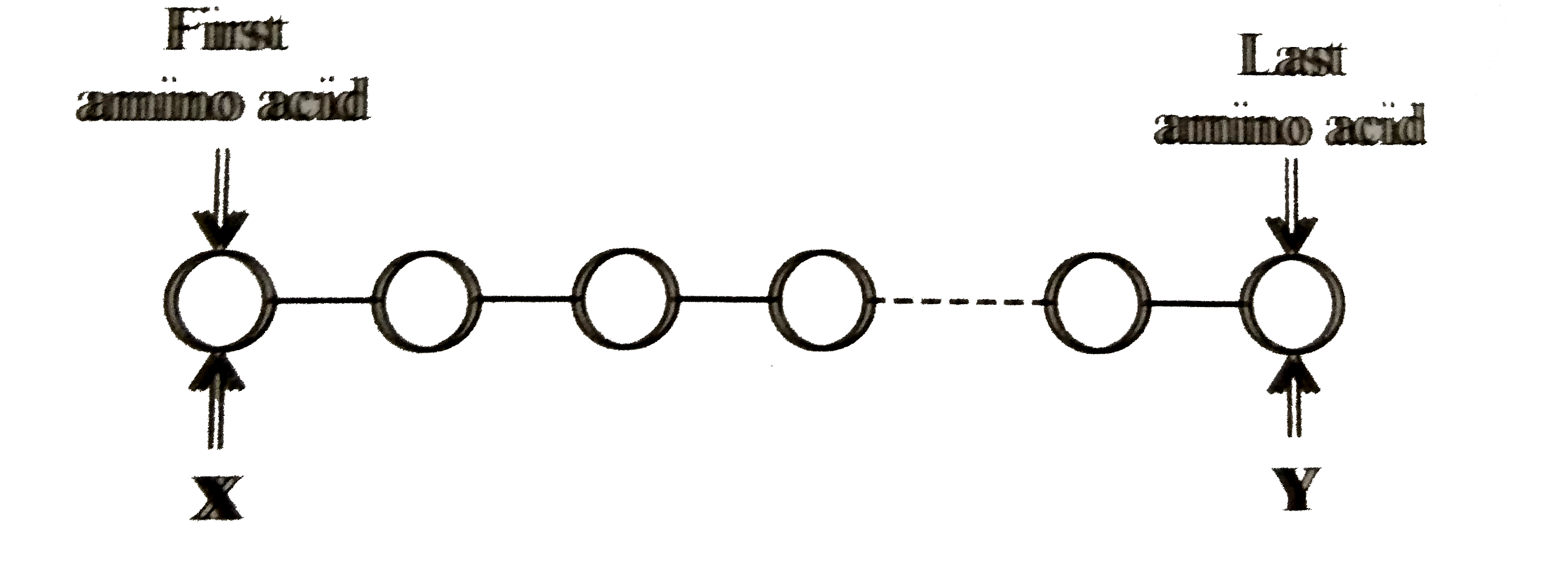 AN- terminal amino acid C- terminal amino acidBN- terminal amino acid N- terminal amino acidCC- terminal amino acid N- terminal amino acidDC- terminal amino acid C- terminal amino acid
AN- terminal amino acid C- terminal amino acidBN- terminal amino acid N- terminal amino acidCC- terminal amino acid N- terminal amino acidDC- terminal amino acid C- terminal amino acidIdentify X and Y in the given sequence.
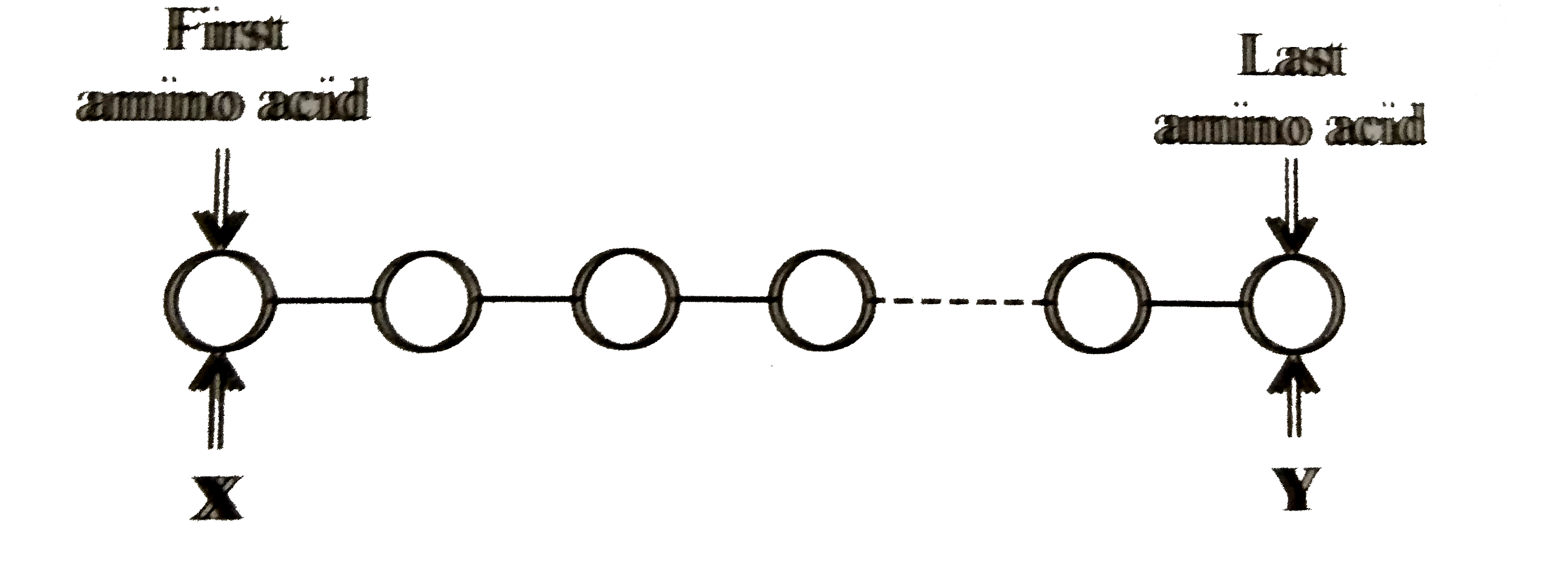 AN- terminal amino acid C- terminal amino acidBN- terminal amino acid N- terminal amino acidCC- terminal amino acid N- terminal amino acidDC- terminal amino acid C- terminal amino acid
AN- terminal amino acid C- terminal amino acidBN- terminal amino acid N- terminal amino acidCC- terminal amino acid N- terminal amino acidDC- terminal amino acid C- terminal amino acidUnder which conditions is xyx−y negative?
A0<y<xBx<y<0Cx<0<yDy<x<0
Identify terms and factors in the expressions given below:
−4x+5y
View SolutionIf x and y are directly proportional, find the values of x1,x2andy1 in the table given below:

View SolutionEqual weights of two substances X and Y are dissolved in equal volumes of water. The osmotic pressure of the solution containing Y is five times the osmotic pressure of the solution containing X. What is the molecular weight of X if that of Y is 60?
View SolutionFrom the below given figure identify X and Y in the given food chain.
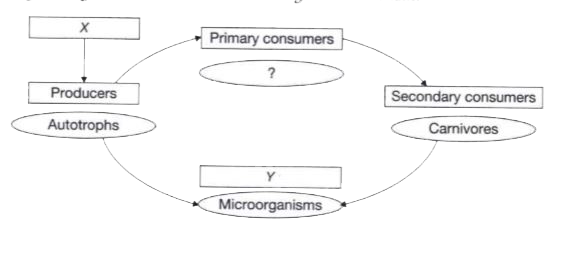
View SolutionObserve the diagram given below and answer the question that follows:
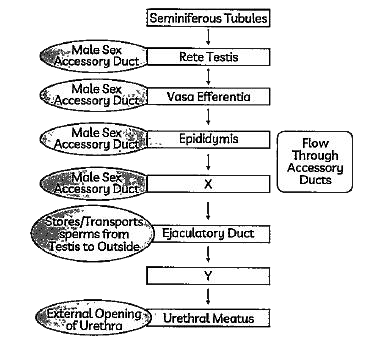
Identify X and Y:
View Solution