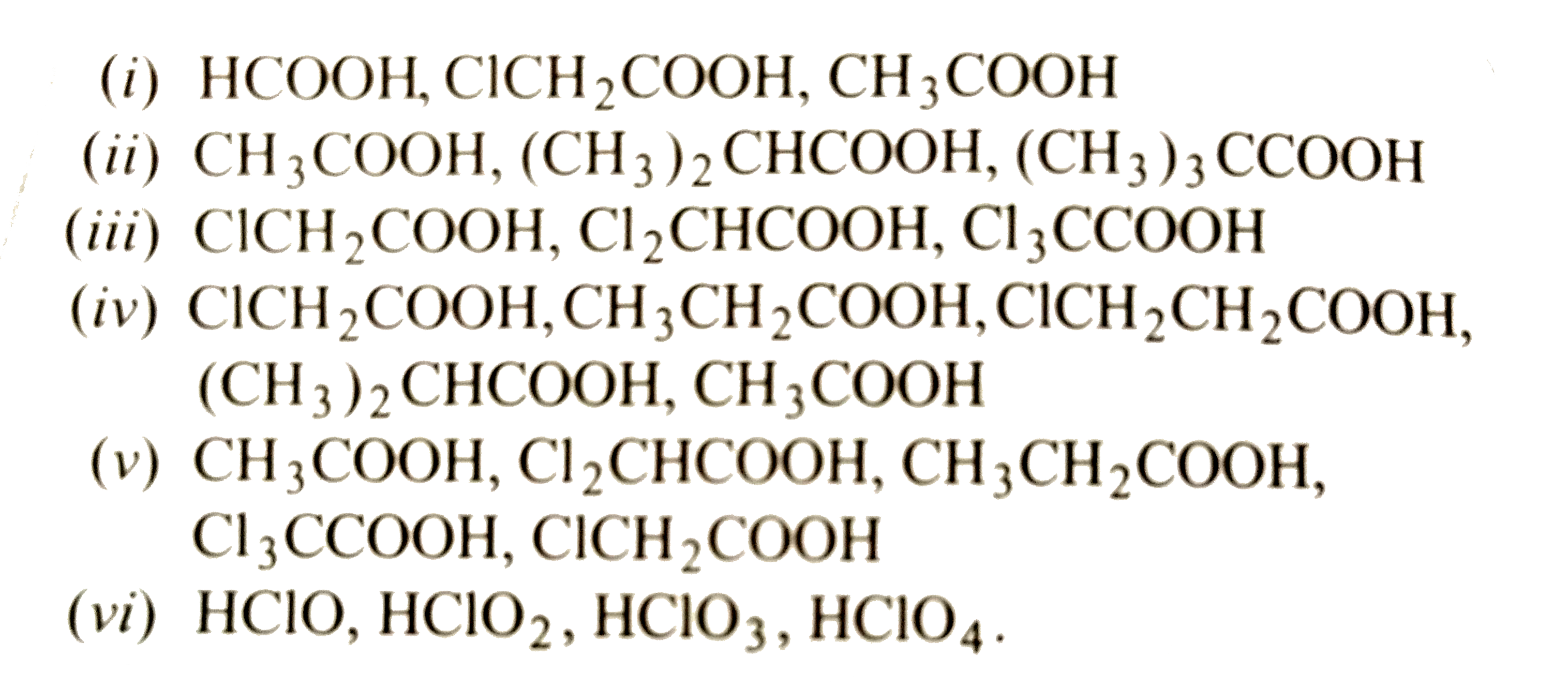Arrange
the
following in the
order of their
increasing
pH
values:
A)Acetic
acid
B)sulphuric acid
C)sodium hydroxide
D)ammonium
hydroxide
Step by step video & image solution for Arrange the following in the order of their increasing pH values: A)Acetic acid<BR> B)sulphuric acid C)sodium hydroxide D)ammonium hydroxide by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Arrange the following acids in order of their increasing acidity
 AA<B<C<DBB<C<A<DCC<B<D<ADC<D<B<A
AA<B<C<DBB<C<A<DCC<B<D<ADC<D<B<AArrange the following acid in order of increasing acidity
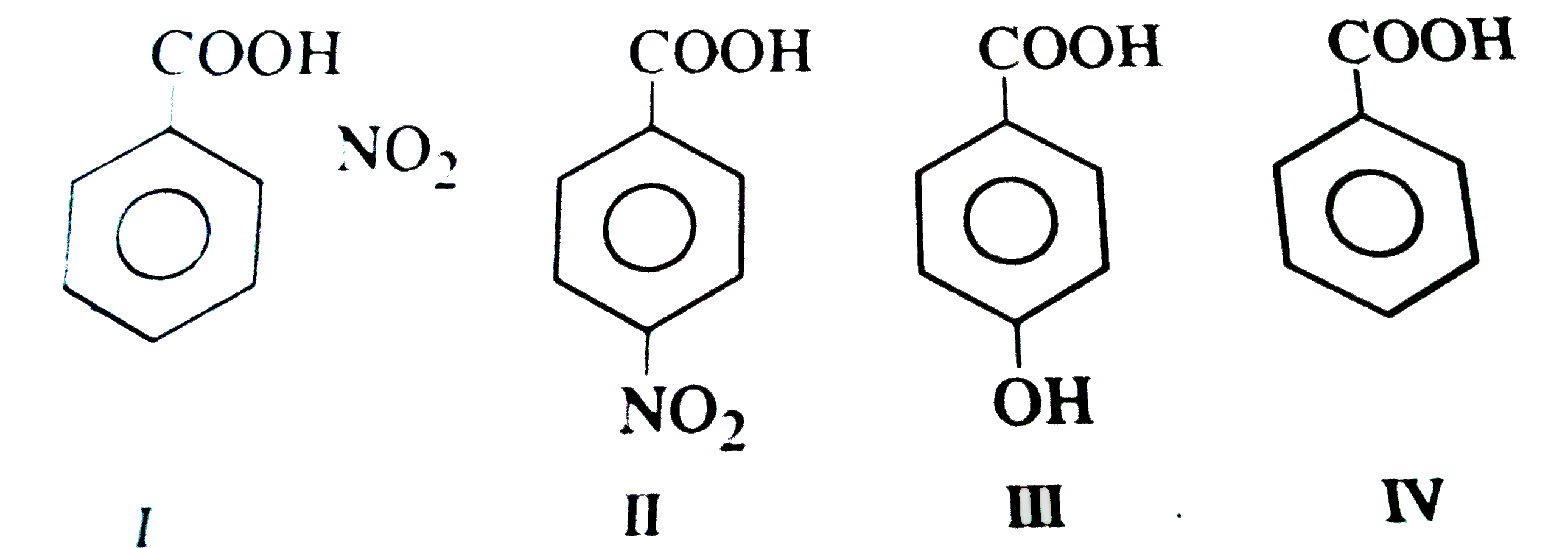 AI>II>III>IVBII>III>I>IVCIII>II>IV>IDIII>IV>II>I
AI>II>III>IVBII>III>I>IVCIII>II>IV>IDIII>IV>II>IArrange the following in increasing order of their acidity? o-cresol(a), salicyclic acid(b), phenol( c)
Ac<a<bBb<c<aCa<b<aDa<c<b
Is sodium hydroxide an acid or a base ?
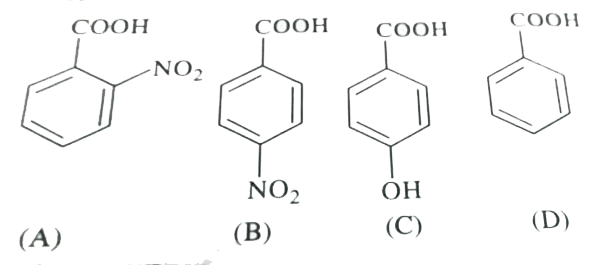
View SolutionArrange the following acids in order of the increasing acidity: A= o-nitro benzoic acid, B = p-nitro benzoic acid, C= p-hydroxy benzoic acid, D= benzoic acid
View SolutionArrange hydroxides of group 2 elements in order of increasing basicity.
View SolutionArrange the following acids in the decreasing order of their basicities.
(A) Phosphoric acid
(B) Hydrochloric acid
(C) Sulphurous acid
View SolutionArrange the following acids in the decreasing order of their basicities.
(A) Phosphoric acid
(B) Hydrochloric acid
(C) Sulphurous acid
View Solution