At a given temperature the internal energy of a substance
in liquid state is equal to that in gaseous state
in liquid state is less than that in gaseous state
in liquid state is more than that in gaseous state
is equal for the three states of matter
The correct Answer is:B
Step by step video, text & image solution for At a given temperature the internal energy of a substance by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
At a given temperature internal energy of a monoatomic, diatomic and non - linear gas is (U0 each. Find their translational and rotiational kinetic energies separately.
View SolutionA substance is taken through the process abc as shown in, if the internal energy of the substance increase by 5000 J and a heat of 2625 cal is given to the system, calculate the value of J.
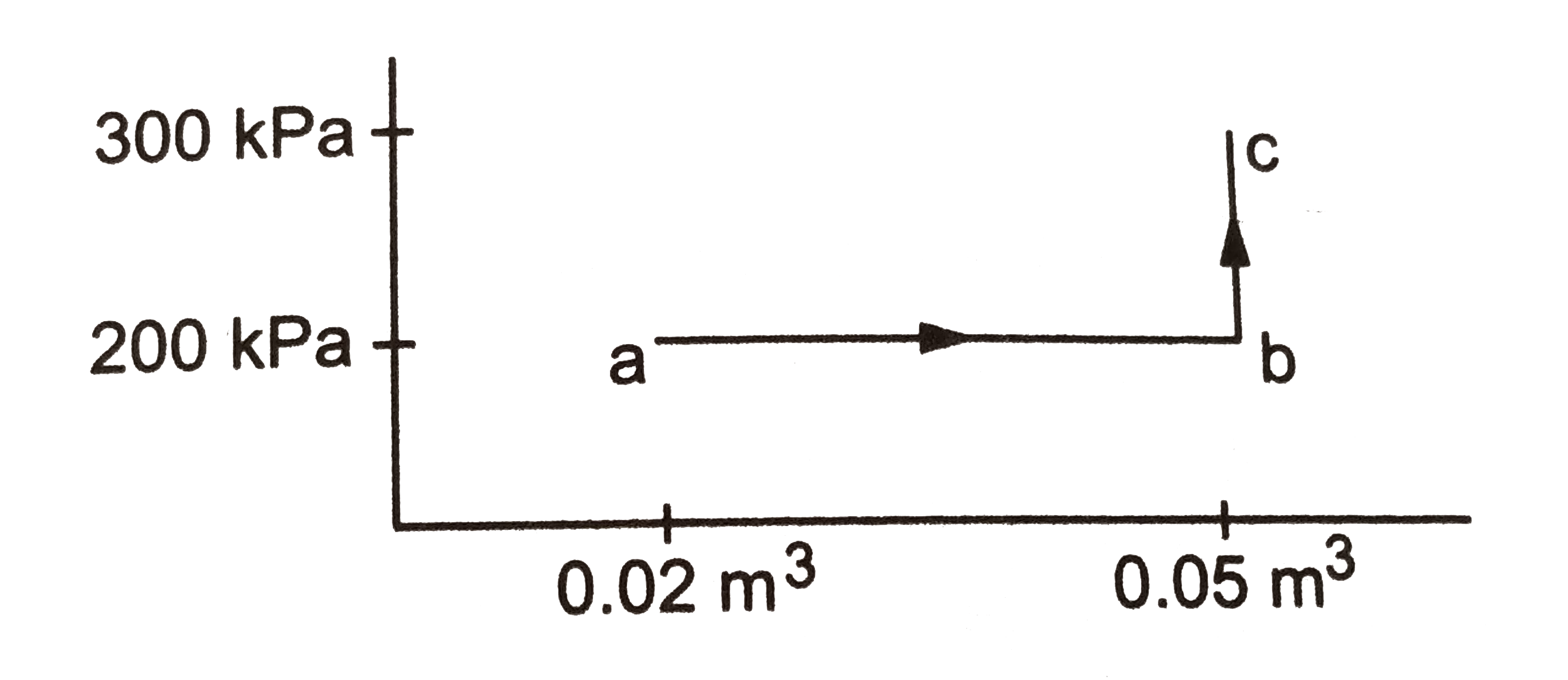
View Solution
Knowledge Check
The change in the internal energy of a substance
AIncrease with increase in temperatureBDecrease with increase in temperatureCCan be calculated the relation E=mc2DRemains unaffected with change in temperatureIn which of the following states the internal energy of a substance in the lowest ?
AGaseousBLiquidCSolidDNone of theseIn which of the following states the internal energy of a substance in the lowest ?
AGaseousBLiquidCSolidDNone of these
Which of the following is correct regarding the internal energy of a substance ?
View SolutionAt a given temperature, the energy of activation of two reaction is same if
View SolutionAt a given temperature, the energy of activation of two reactions is same if
View Solution3 mole of O2 mixed with 5 moles of Argon at temperature T. Find total internal energy of system
View SolutionAt high temperature, the molecules of a substance
View Solution
