An atom has electronic configuration 1s22s22p63s23P63d34s2 . In which group wouold it be placed ?
Fifth
Fifteenth
Second
Third
The correct Answer is:A
The electronic configuration clearly suggest that it is a d-block element (having configuration (n−1)d1−10ns0−2) which starts from III B and goes till II B. Hence with d3 configuration it would be classified in the group.
Topper's Solved these Questions
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBOOK - DISHA PUBLICATIONCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESEXERCISE - Exercise -1 :Concept Builder (Topic 2)
22 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBOOK - DISHA PUBLICATIONCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESEXERCISE - Exercise -1 :Concept Builder (Topic 3)
17 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBOOK - DISHA PUBLICATIONCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESEXERCISE - Exercise -2 : Concept Applicator
1 Video
- CHEMISTRY IN EVERDAY LIFE BOOK - DISHA PUBLICATIONCHAPTER - CHEMISTRY IN EVERDAY LIFE EXERCISE - Exercise
88 Videos
- COORDINATION COMPOUNDS BOOK - DISHA PUBLICATIONCHAPTER - COORDINATION COMPOUNDS EXERCISE - Exercise
122 Videos
Similar Questions
The correct order of radii is
View SolutionElectron affinity is maximum for
View SolutionAmong Al2O3,SiO2,P2O3 and SO2 the correct order of acidic strength is:
View SolutionThe correct match between items of List - I and List - II is :
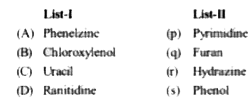
View SolutionWhich of the following complexes will show geometrical isomerism ?
View SolutionAn atom has electronic configuration 1s22s22p63s23P63d34s2 . In which group wouold it be placed ?
View SolutionAn atom has electronic configuration 1s22s22p63s23P63d34s2 . In which group wouold it be placed ?
View Solutionकुछ तत्वों के इलेक्ट्रॉनिक विन्यास नीचे दिये गये है। इन तत्वों को आवर्त सारणी में किस समूह और किस आवर्त में स्थान दिया जायेगा और क्यों?
1s2
(b) 1s2,2s22p6,3s23p6
(c ) 1s2,2s22p6,3s23p3
(d) 1s2,2s22p6
(e) 1s2,2s22p6,3s23p63d10,4s24p6
(f) 1s2,2s22p6,3s23p6,4s2
View SolutionAn atom has electronic configuration 1s22s22p63s23p63d34s2, you will place it in which group ?
View SolutionQ .Which of the following electron configurations is correct for nickel (atomic number "28" )? a) 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(1)3d^(9) (b) 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(8) (c) 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(0)3d^(10)
View SolutionThe element with electronic configuration of its atom 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)3d^(10)4s^(1) is:
View SolutionConsider the following list of electron configurations:
(1) 1s22s23s2 (2) 1s22s22p6
(3) 1s22s22p63s1 (4) 1s22s22p63s23p64s2
(5) 1s22s22p63s23p64s23d6
Which one of the above configuration represents a transition element?
View Solutionयदि एक परमाणु का एलेक्ट्रोनीक विन्यास 1s22s22p63s23p3 है तो इसे रखा जायेगा
View SolutionIf an atom has electronic configuration 1s^(2)2s^(2), 2p^(6) 3s^(2) 3p^(6) 3d^(3) 4s^(2) It will be placed in
View SolutionThe position of the element with electronic configuration 1s^(2) 2s^(2) 2p^(6) 3s^(2) 3p^(6) 3d^(3) 4s^(2) is placed in the group ….. .
View SolutionAn atom has electronic configuration 1s22s22p63s23P63d34s2 . In which group wouold it be placed ?
View Solution

