The incorrect statement among the following is
The first ionization potential of Al is less than the first ionization potential of Mg.
The second ionization potential of Mg is greater than the second ionization potential of Na.
The first ionization potential of Na is less than the first ionization potential of Mg.
The third ionization potential of Mg is greater than the third ionization potential of Al
The correct Answer is:B
IE2 of Mg is lower than that of a Na because in case of Mg+, 3s-electron has to be removed whereas in case of Na+ an electron is removed from the stable inert gas configuration which is difficult.
Topper's Solved these Questions
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBOOK - DISHA PUBLICATIONCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESEXERCISE - Exercise -2 : Concept Applicator
1 Video
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBOOK - DISHA PUBLICATIONCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESEXERCISE - Exercise -1 :Concept Builder (Topic 3)
17 Videos
- CHEMISTRY IN EVERDAY LIFE BOOK - DISHA PUBLICATIONCHAPTER - CHEMISTRY IN EVERDAY LIFE EXERCISE - Exercise
88 Videos
- COORDINATION COMPOUNDS BOOK - DISHA PUBLICATIONCHAPTER - COORDINATION COMPOUNDS EXERCISE - Exercise
122 Videos
Similar Questions
Among Al2O3,SiO2,P2O3 and SO2 the correct order of acidic strength is:
View SolutionElectron affinity is maximum for
View SolutionThe correct match between items of List - I and List - II is :
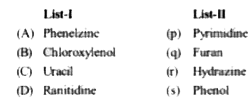
View SolutionWhich of the following complexes will show geometrical isomerism ?
View SolutionThe incorrect statement(s) among the following is/are?
View SolutionThe incorrect statement among the following are
View SolutionThe incorrect statement among the following
View SolutionAmong the following, the INCORRECT statement is
View SolutionOne among the following is an incorrect statement
View SolutionAmong the following, the incorrect statement is :
View SolutionThe incorrect statement among the following is
View SolutionThe INCORRECT statement among the following is __________ .
View SolutionWhich statement is INCORRECT among the following ?
View SolutionAmong the following the incorrect statement is
View SolutionAmong the following statements the incorrect is/are :
View SolutionAmong the following statements, incorrect statement is
View Solution

