Which of the following would have a permanent dipole moment ?
A
SiF4
B
SF4
C
XeF4
D
BF3
Video Solution
Text Solution
The correct Answer is:B
SF4 has permanent dipole moment. SF4 has sp3d hybridization and see saw shape (irregular geometry)
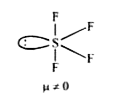
Whereas XeF4 shows squre planar geometry, SiF4 has tetrahedral shape, and BF3 has triangular planar shape. All these are symmetric molecules. Hence μ=0
|
Similar Questions
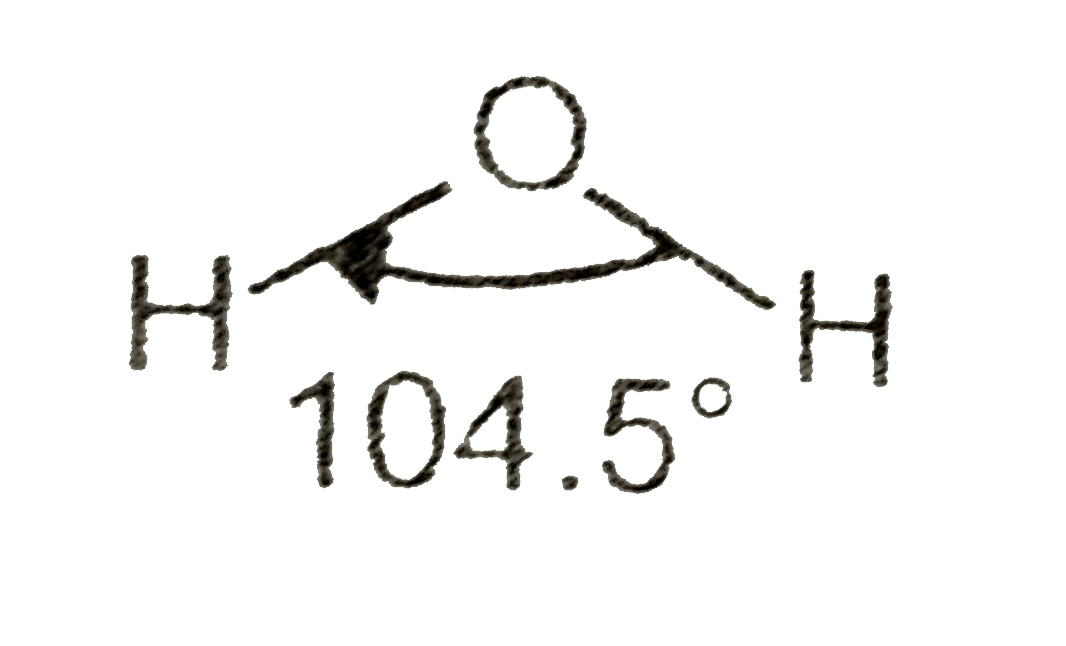
Electric Dipole moment is a vector quantify . If a compound contain more than one polar bond then , the net electric dipole moment is equal to vector sum of individual dipole moment .
e.g., In H2O
μ=1.84D
Which of the following compounds have zero dipole moment ?
View SolutionWhich of the following molecules will have a peranent dipole moment ?
View Solution
Knowledge Check
Which of the following would have permanent dipple moment ?
ASF4BXeF4CSiF4DBF3Which of the following molecules have permanent dipole moment ?
AH2OBBrF5CXeO2F2DAll of theseWhich of the given compound have a permanent dipole moment ?
AXeF4BSiF4CBF3DSF4
Which of the following will have largest dipole moment?
View SolutionWhich of the following compounds have finite dipole moments ?
View SolutionWhich of the following will have the highest dipole moment ?
View SolutionWhich of the following compounds have zero dipole moment ?
View SolutionWhich of the following molecule have zero dipole moment ?
View Solution

