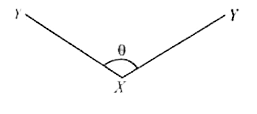
Which bond angle, θ would result in the maximum dipole moment for the triatomic molecule XY2 ?
A
θ=90∘
B
θ=120∘
C
θ=150∘
D
θ=180∘
Video Solution
Text Solution
The correct Answer is:A
The dipole moment of two dipoles inclined at an angle θ is given by the equation μ=√X2+Y2+2XYcosθcos90∘=0 Since the angle increases from 90∘ to 180∘ the value of cosθ becomes more and more - ve and hence resultant dipole moment decreases. Thus, dipole moment is maximum when θ=90∘.
|
Similar Questions
Knowledge Check
Which bond angle θ would result in the maximum dipole moment for the triatomic YXY?
Aθ=90∘Bθ=120∘Cθ=150∘Dθ=180∘Which bond angle, θ would result in the maximum dipole moment for the triatomic molecule XY2 ?
Aθ=90∘Bθ=120∘Cθ=150∘Dθ=180∘Which bond angle 0 would result in the maximum dipole moment for the triatomic molecule XY2 as shown in figure?
 A90∘B120∘C150∘D180∘
A90∘B120∘C150∘D180∘

