In the given pair compounds in which pair second cpmpound has highter boiling point than first compund?
A
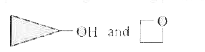
B
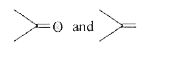
C
HO−CH2−CH2−OHandCH3−CH2−CH2−OH
D
Video Solution
Text Solution
The correct Answer is:d
B.P ∝ extent of intermolecular H - bonding ∝ dipole - dipole attraction or H - bonding.
|
Similar Questions
Knowledge Check
In the given pair compounds in which pair second cpmpound has highter boiling point than first compund?
A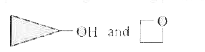 B
B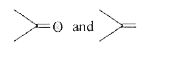 CHO−CH2−CH2−OHandCH3−CH2−CH2−OHD
CHO−CH2−CH2−OHandCH3−CH2−CH2−OHD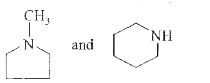
Among the given pairs in which pairs first compound has higher boiling point than second?
ACH3−⊕CH−CH3andCH3−⊕CH−OCH3BCH3−CH2−CH2−CH3andCH3−CH2−CH3CCH3−CH2−CH2−CH2−CH3andCH3−CH2−CH3∣C−H−CH2−OHDCH3−CH2−CH2−CH3andCH3−CH2−CH2−CIWhich compound has the highest boiling point
AAcetoneBDiethyl etherCMethanolDEthanol
Among the given pairs, in which pair secod compound has less enol content than first compound?
View SolutionIn which case second has lower boiling point than first ?
View SolutionAmong the given pairs, in which pair second compound has less enol content:
View SolutionWhich of the following compound has highest boiling point ?
View SolutionWhich of the following compounds has highest boiling point ?
View Solution
