Arrange the following compounds in order of increasing solubility
(i) MgF2 (ii) CaF2 (iii) BaF2
A
(i)<(ii)<(iii)
B
(ii)<(i)<(iii)
C
(ii)<(iii)<(ii)
D
(iii)<(ii)<(i)
Text Solution
The correct Answer is:A
|
Similar Questions
Knowledge Check
Arrange the following compounds in the order of decreasing acidity.
I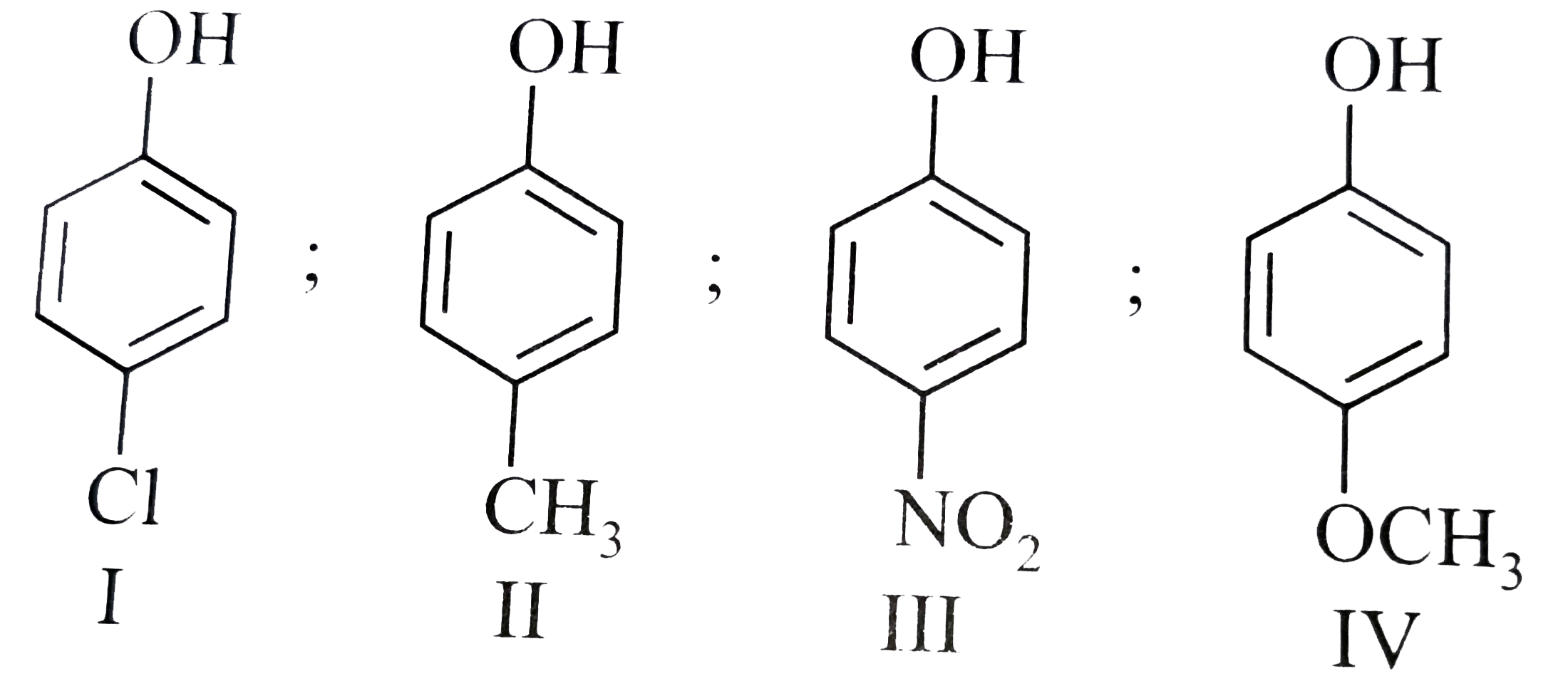 II
II  III
III  IV
IV  AII>IV>I>IIIBI>II>III>IVCIII>I>II>IVDIV>III>I>II
AII>IV>I>IIIBI>II>III>IVCIII>I>II>IVDIV>III>I>IIArrange the following compounds in order of increasing dipole moment:
(I) Toluene
(II) m-Dichlorobenzene
(III) o-Dichlorobemzene
(IV) p-DichlorobenzeneAI<IV<II<IIIBIV<I<II<IIICIV<I<III<IIDIV<II<I<III
Arrange the following compounds in the order of increasing boiling points :
(i)CH3CN (ii)CH3Cl (iii)CH3NC
View SolutionArrange the following in increasing order of their
(i) Solubility and (ii) Boiling point
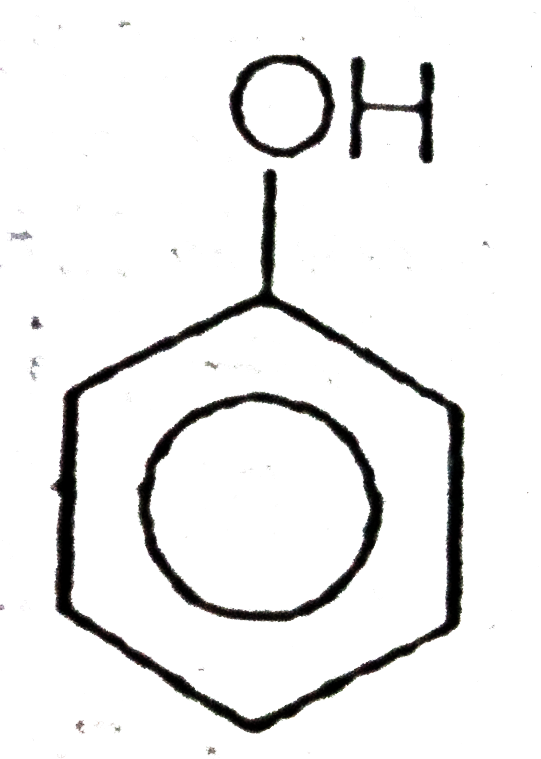
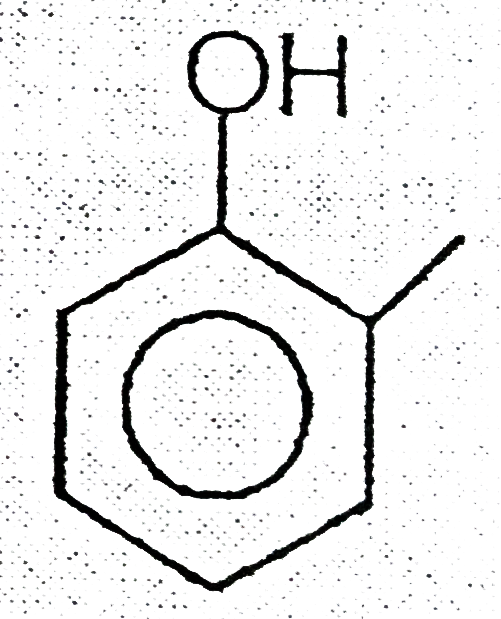
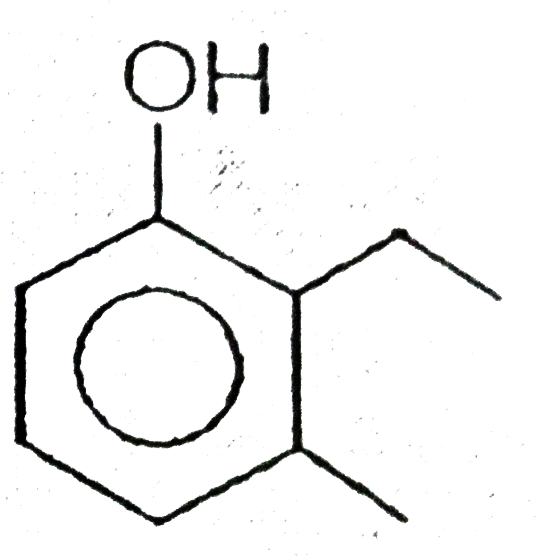
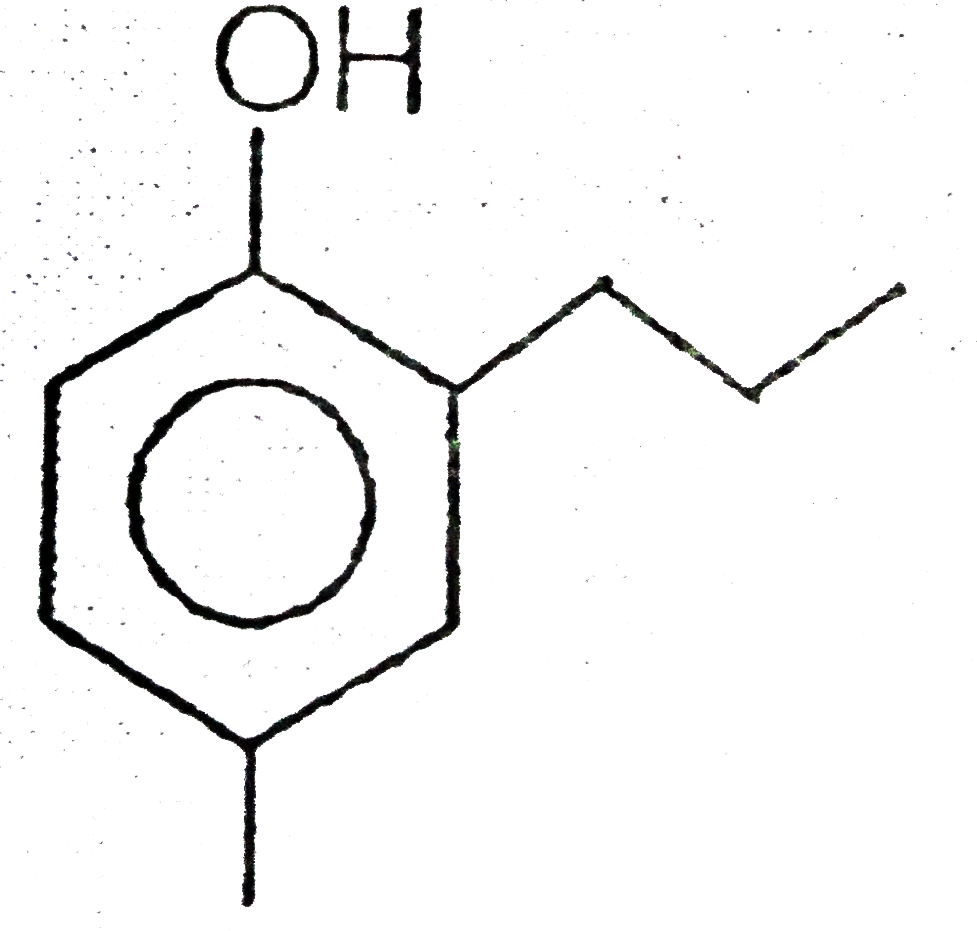
View SolutionArrange the following in increasing order of their
(i) Solubility and (ii) Boiling point

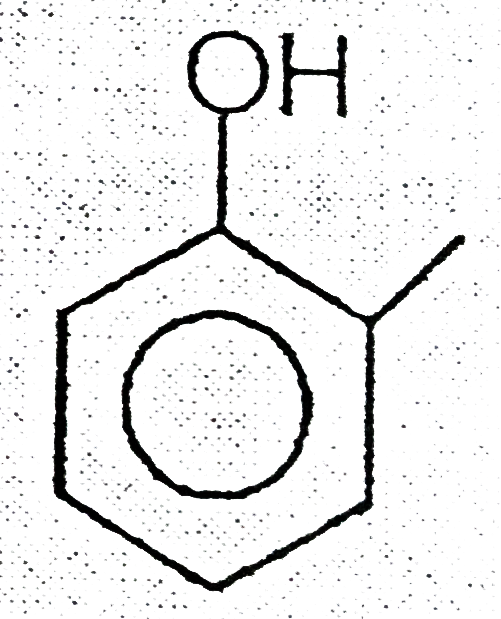


View SolutionArrange the following in the increasing order of acidic strength
(i) FSO3H (ii) HCO−3 (iii) H2O (iv) HSO−4
View SolutionArrange the following in the increasing order of acidic strength
(i) FSO3H (ii) HCO−3 (iii) H2O (iv) HSO−4
View SolutionArrange the following in increasing order of their
(i) Solubility and , (ii) Boiling point
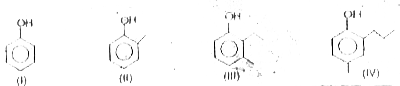
View Solution

