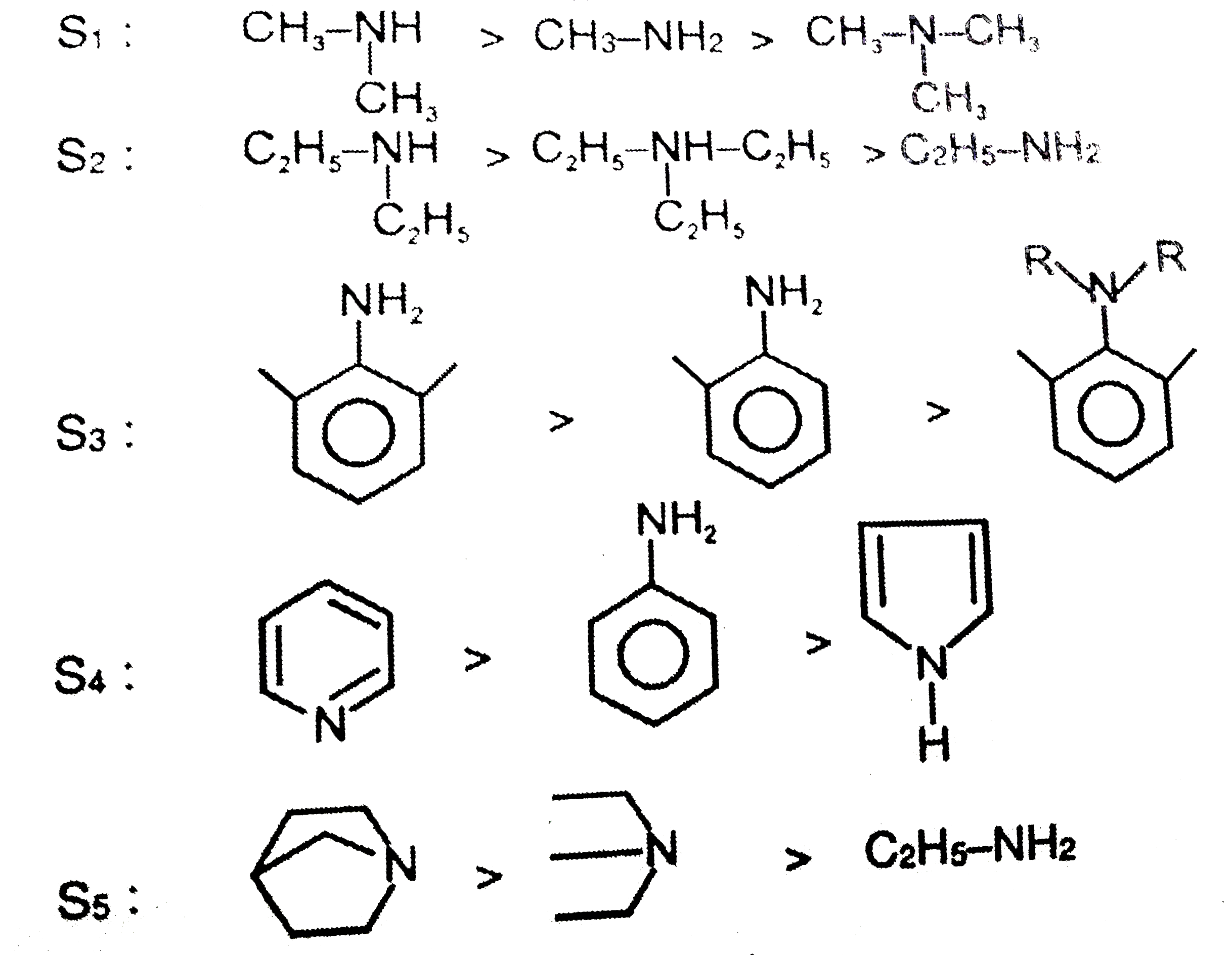Which of the following is/are correct for basic strength:
A
MgO<BeO<CaO<BaO
B
BeO<MgO<CaO<BaO
C
BaO<CaO<MgO<BeO
D
CaO<BaO<BeO<MgO
Video Solution
Text Solution
The correct Answer is:B
The basic character of oxides increases down the group.
|
Similar Questions
Knowledge Check
Which of the following is correct order of basic strength for the given compounds ?
(i)CH3−CH3−C−NH2∣∣NH (ii)CH3−C−CH3∣∣NH
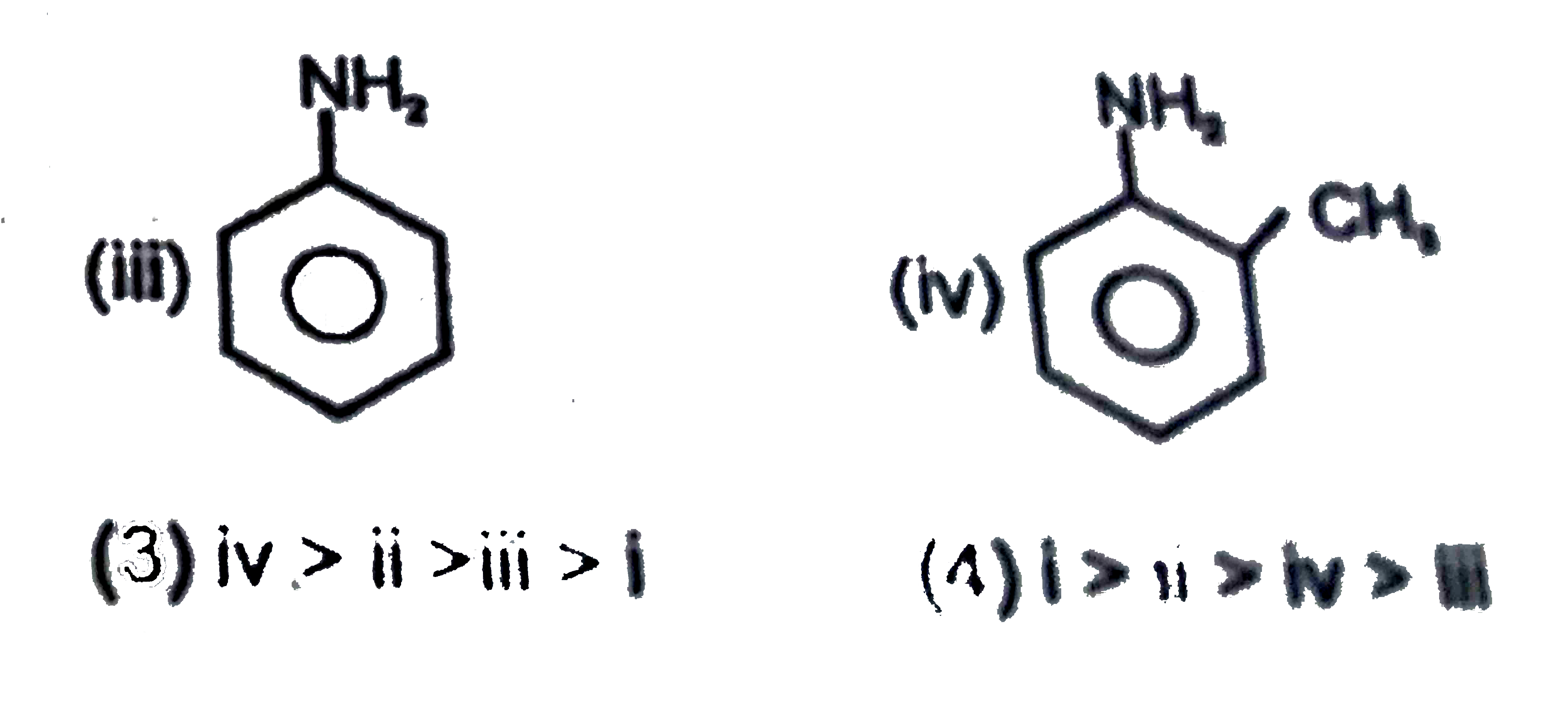 AI>ii>iii>ivBiv>iii>ii>iCiv>ii>iii>iDI>ii>iv>iii
AI>ii>iii>ivBiv>iii>ii>iCiv>ii>iii>iDI>ii>iv>iiiWhich of the following is/are correct for basic strength:
AMgO<BeO<CaO<BaOBBeO<MgO<CaO<BaOCBaO<CaO<MgO<BeODCaO<BaO<BeO<MgOWhich of the following has correct increasing basic strength?
AMgO<BeO<CaO<BaOBBeO<MgO<CaO<BaOCBaO<CaO<MgO<BeODCaO<BaO<BeO<MgO
Which of the following is (are) correct order(s) for basic strength ?
View SolutionWhich of the following order for basic strength is/are correct?
View SolutionWhich of the following is the correct order of basicity?
View SolutionWhich of the following is the correct order of acidic strength ?
View Solution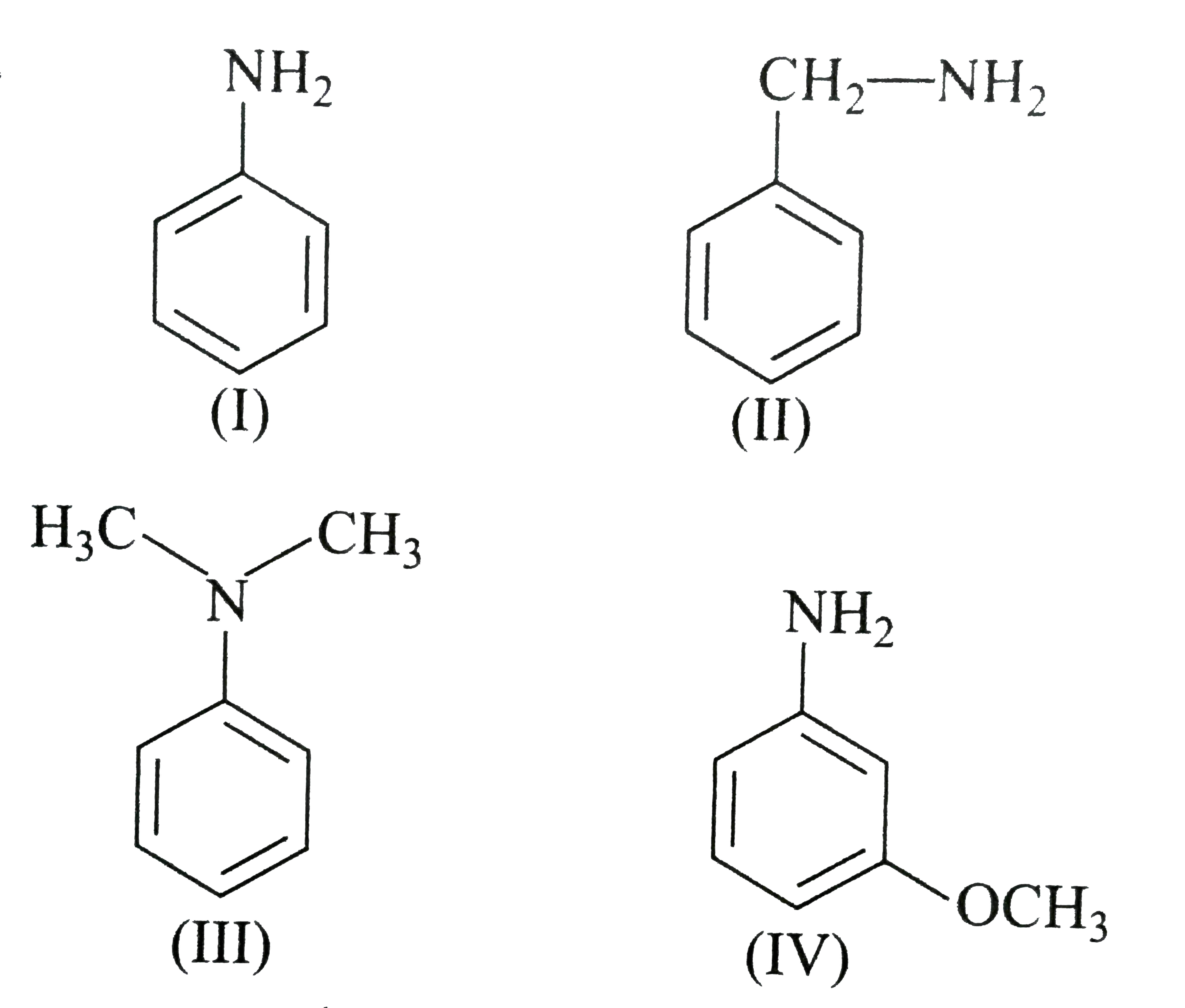
Correct order of basic strength
View Solution