The pressure on a sample of water at its triple point is reducend while the temperature is held contant .Which phases changes are favoured?
(1) melting of ice
(2)sublimation of ice
(3) vaporization of liquid water
I only
III only
II only
II and III
The correct Answer is:D
Pressure ↓⇒ towards more volume.
Similar Questions
Define triple point of a substance. Write its value of temperature and pressure for water.
View Solution(1) Take a few pieces of ice in a glass beaker as shown in figure.
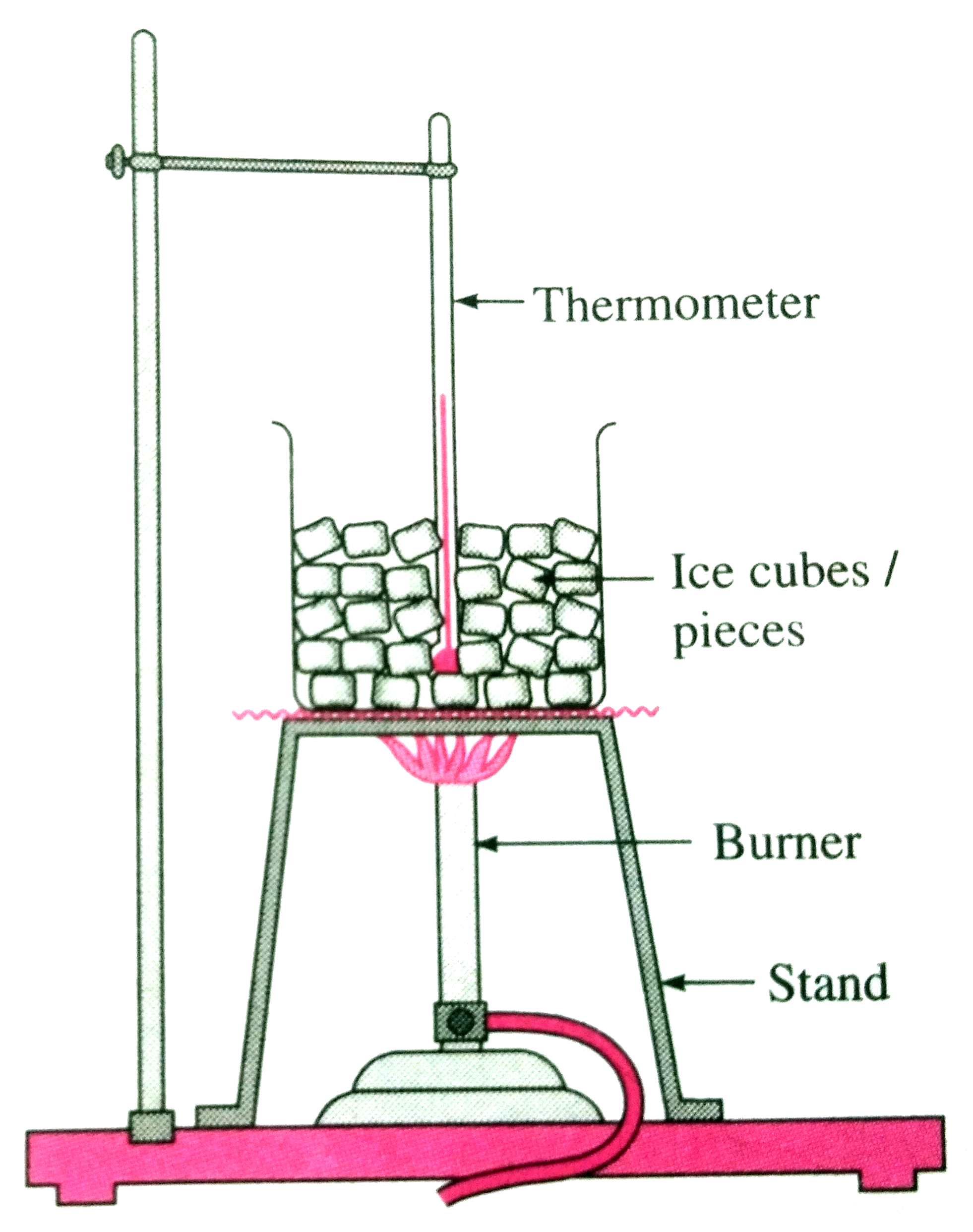
(2) Insert the bulb of a thermometer in ice and measure its temperature.
(3) Put the beaker on a stand and heat the ice using a burner.
(4) Record the temperature using the thermometer after every minute.
(5) As the ice is heated,it starts melting. Stir the mixture of ice and water.
(6) Continue the heating even after ice starts melting.
(7) Draw a graph of temperature versus time.
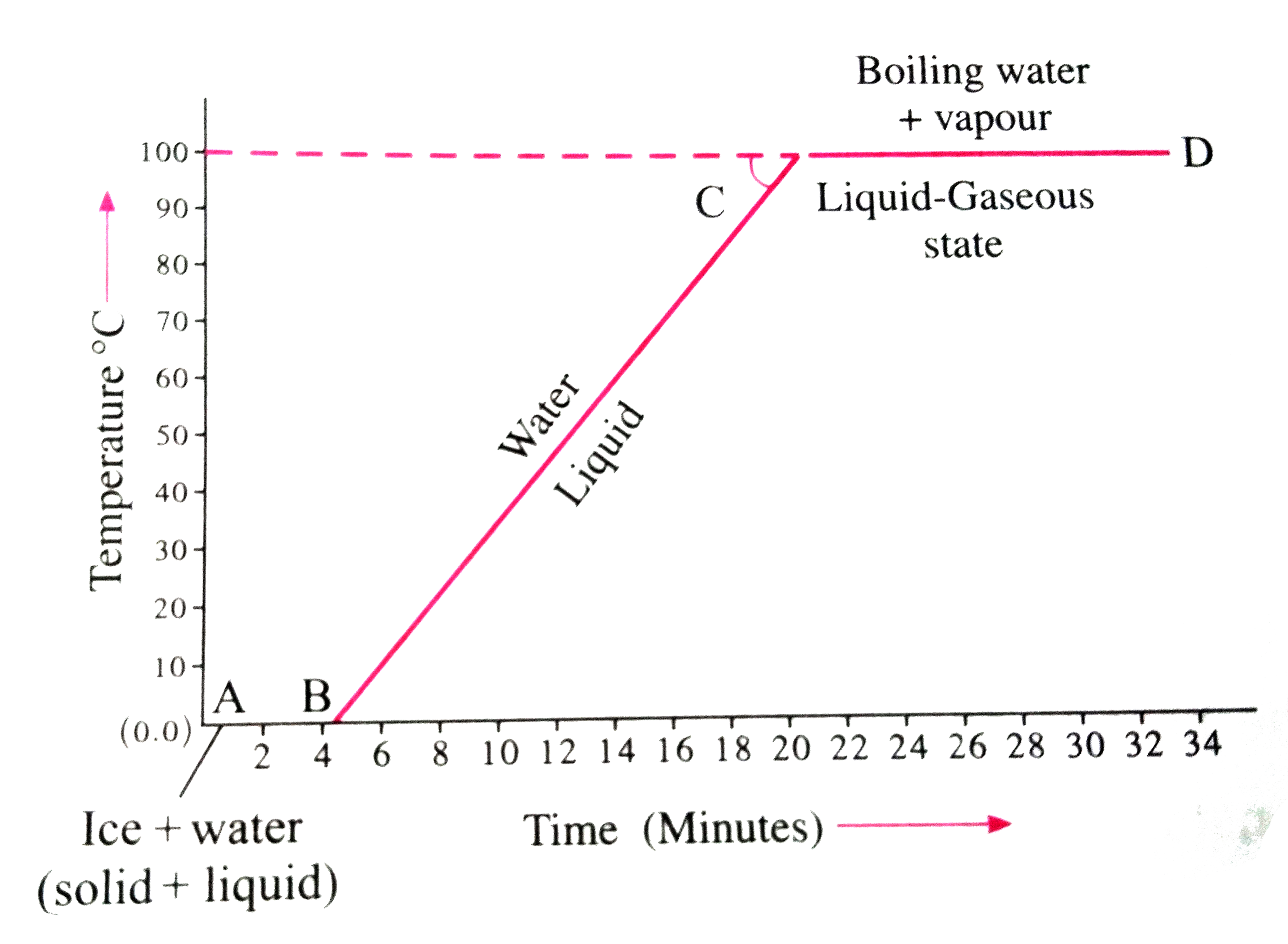
Define boiling point of a liquid. OR What is boiling point of a liquid ?
View Solution
Knowledge Check
The pressure on a sample of water at its triple point is reducend while the temperature is held contant .Which phases changes are favoured?
(1) melting of ice
(2)sublimation of ice
(3) vaporization of liquid waterAI onlyBIII onlyCII onlyDII and IIIAt its melting point ice is lighter than water because
AH2O molecules are more closely packed in solid state.Bice crystals have hollow hexagonal arrangement of H2O molecules.Con meltign of ice the H2O molecules shrinks in size.Dice forms mostly heavy water on first melting.At its melting point ice is lighter than water because
AH2O molecules are more closely packed in solid stateBice crystals have hollow hexagonal arrangement of H2O molecules.Con melting of ice the H2O molecules shrinks in sizeDice froms mostly heavy water on first melting.
At its melting point ice is lighter than water because
View SolutionAt its melting point ice is lighter than water because
View SolutionAt its melting point ice is lighter than water becouse
View SolutionAs its melting point, ice is lighter than water because:
View SolutionThe temperature and pressure at which ice, liquid water and water vapour can exist together are
View Solution

