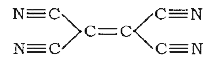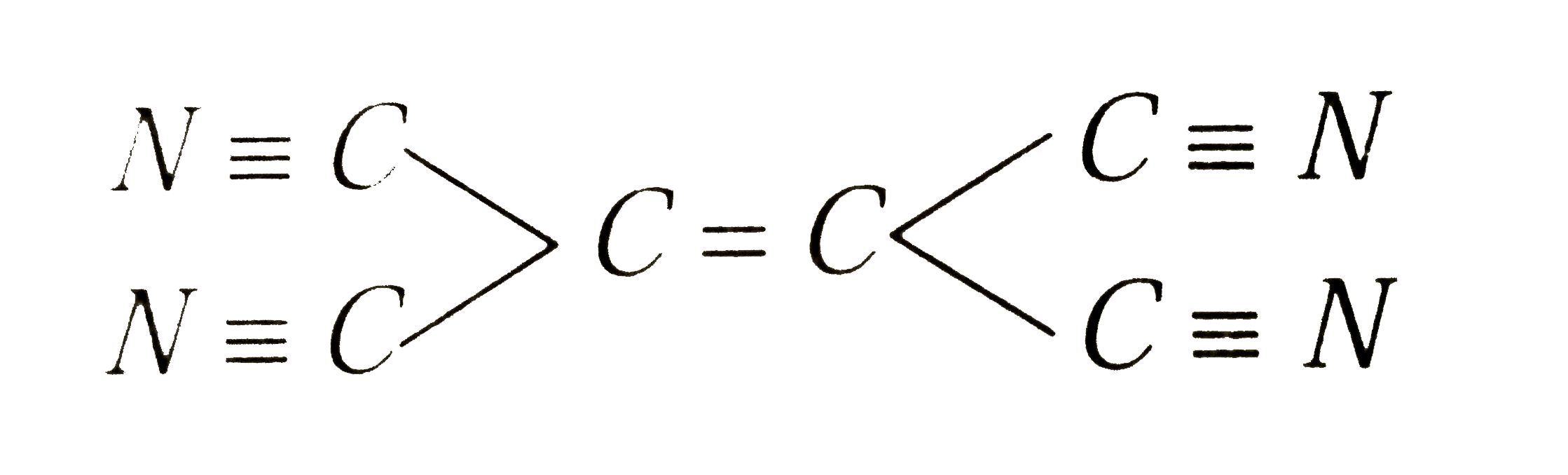How many σandπ bonds are there in the molecule of tetracyanoethene ?
(NC)2C=C(CN)2
9σ,7π
5σ,9π
5σ,8π
9σ,9π
The correct Answer is:D
To determine the number of sigma (σ) and pi (π) bonds in tetracyanoethene (NC)₂C=C(CN)₂, we can follow these steps:
Step 1: Draw the Structure
Tetracyanoethene has the following structure:
N≡C|C=C|C≡N
In this structure, we can see that there are two cyano (CN) groups attached to each carbon atom of the double bond.
Step 2: Identify the Bonds
1. Double Bond (C=C):
- Contains 1 sigma bond and 1 pi bond.
2. Triple Bonds (C≡N):
- Each cyano group (C≡N) contains 1 sigma bond and 2 pi bonds.
- Since there are 2 cyano groups, this contributes:
- 2 sigma bonds (1 for each C≡N)
- 4 pi bonds (2 for each C≡N)
Step 3: Count All Bonds
Now, let's summarize the bonds:
- From the C=C double bond:
- 1 σ bond
- 1 π bond
- From the two C≡N triple bonds:
- 2 σ bonds (1 for each C≡N)
- 4 π bonds (2 for each C≡N)
Step 4: Total Count
Now, we can add up the total number of sigma and pi bonds:
- Total σ bonds:
- From C=C: 1 σ bond
- From C≡N: 2 σ bonds
- Total σ bonds = 1 + 2 = 3 σ bonds
- Total π bonds:
- From C=C: 1 π bond
- From C≡N: 4 π bonds
- Total π bonds = 1 + 4 = 5 π bonds
Final Answer
- Total σ bonds = 3
- Total π bonds = 4
Thus, in tetracyanoethene, there are 3 sigma bonds and 4 pi bonds.
Tetracyanoethene has the following structure:
N≡C|C=C|C≡N
In this structure, we can see that there are two cyano (CN) groups attached to each carbon atom of the double bond.
1. Double Bond (C=C):
- Contains 1 sigma bond and 1 pi bond.
2. Triple Bonds (C≡N):
- Each cyano group (C≡N) contains 1 sigma bond and 2 pi bonds.
- Since there are 2 cyano groups, this contributes:
- 2 sigma bonds (1 for each C≡N)
- 4 pi bonds (2 for each C≡N)
Now, let's summarize the bonds:
- From the C=C double bond:
- 1 σ bond
- 1 π bond
- From the two C≡N triple bonds:
- 2 σ bonds (1 for each C≡N)
- 4 π bonds (2 for each C≡N)
Now, we can add up the total number of sigma and pi bonds:
- Total σ bonds:
- From C=C: 1 σ bond
- From C≡N: 2 σ bonds
- Total σ bonds = 1 + 2 = 3 σ bonds
- From C=C: 1 π bond
- From C≡N: 4 π bonds
- Total π bonds = 1 + 4 = 5 π bonds
- Total σ bonds = 3
- Total π bonds = 4
Topper's Solved these Questions
Similar Questions
Knowledge Check
How many sigma and pi bonds are there in the molecule of tetracyano ethylene ? .
A4σ,14πB5σ,13πC8σ,10πD9σ,9πHow many sigma and pi-bonds are there in the molecule of dicyanoethene (CN−CH=CH−CN)?
A3 sigma and 3 piB5 sigma and 2 piC7 sigma and 5piD2 sigma and 3piHow many σ and π bonds are there in the molecule C2(CN)4?
AFive σ and eightπBNine σ and nine πCNine σ and seven πDFive σ and nine π
How many σ and π bonds are present in the molecule of ethene ?
View SolutionHow many sigma and pi bonds are present in HCN molecule?
View SolutionHow many sigma and pi bonds are present in borazole?
View SolutionHow many sigma and pi bonds are present in toluene ?
View SolutionHow many sigma and pi-bonds are present in toluene?
View Solution