In a body centred cubic arrangement----------- atoms along the body diagonal touch each other.
To solve the question regarding how many atoms along the body diagonal of a body-centered cubic (BCC) arrangement touch each other, we can follow these steps:
1. Understand the Structure of BCC:
- In a body-centered cubic (BCC) arrangement, there are atoms located at each of the eight corners of the cube and one atom at the center of the cube.
2. Identify the Body Diagonal:
- A body diagonal in a cube connects two opposite corners of the cube and passes through the center atom.
3. Visualize the Atoms Along the Body Diagonal:
- When you draw the body diagonal, you will see that it starts from one corner atom, passes through the center atom, and ends at the opposite corner atom.
4. Count the Atoms on the Body Diagonal:
- The body diagonal will touch three atoms:
- The first atom at one corner,
- The center atom,
- The second atom at the opposite corner.
5. Conclusion:
- Therefore, along the body diagonal in a BCC arrangement, 3 atoms touch each other.
Final Answer:
3 atoms along the body diagonal touch each other in a body-centered cubic arrangement.
---
- In a body-centered cubic (BCC) arrangement, there are atoms located at each of the eight corners of the cube and one atom at the center of the cube.
- A body diagonal in a cube connects two opposite corners of the cube and passes through the center atom.
- When you draw the body diagonal, you will see that it starts from one corner atom, passes through the center atom, and ends at the opposite corner atom.
- The body diagonal will touch three atoms:
- The first atom at one corner,
- The center atom,
- The second atom at the opposite corner.
- Therefore, along the body diagonal in a BCC arrangement, 3 atoms touch each other.
3 atoms along the body diagonal touch each other in a body-centered cubic arrangement.
Similar Questions
Potassium crystallizes in "body centred cubic" arrangement and the surfaces of adjacent atom along the edges of unit cells are 71.4 picometre apart. Determine the density of metal. Atomic weight of K = 39.
View SolutionA body centred cubic arrangement is shown below:
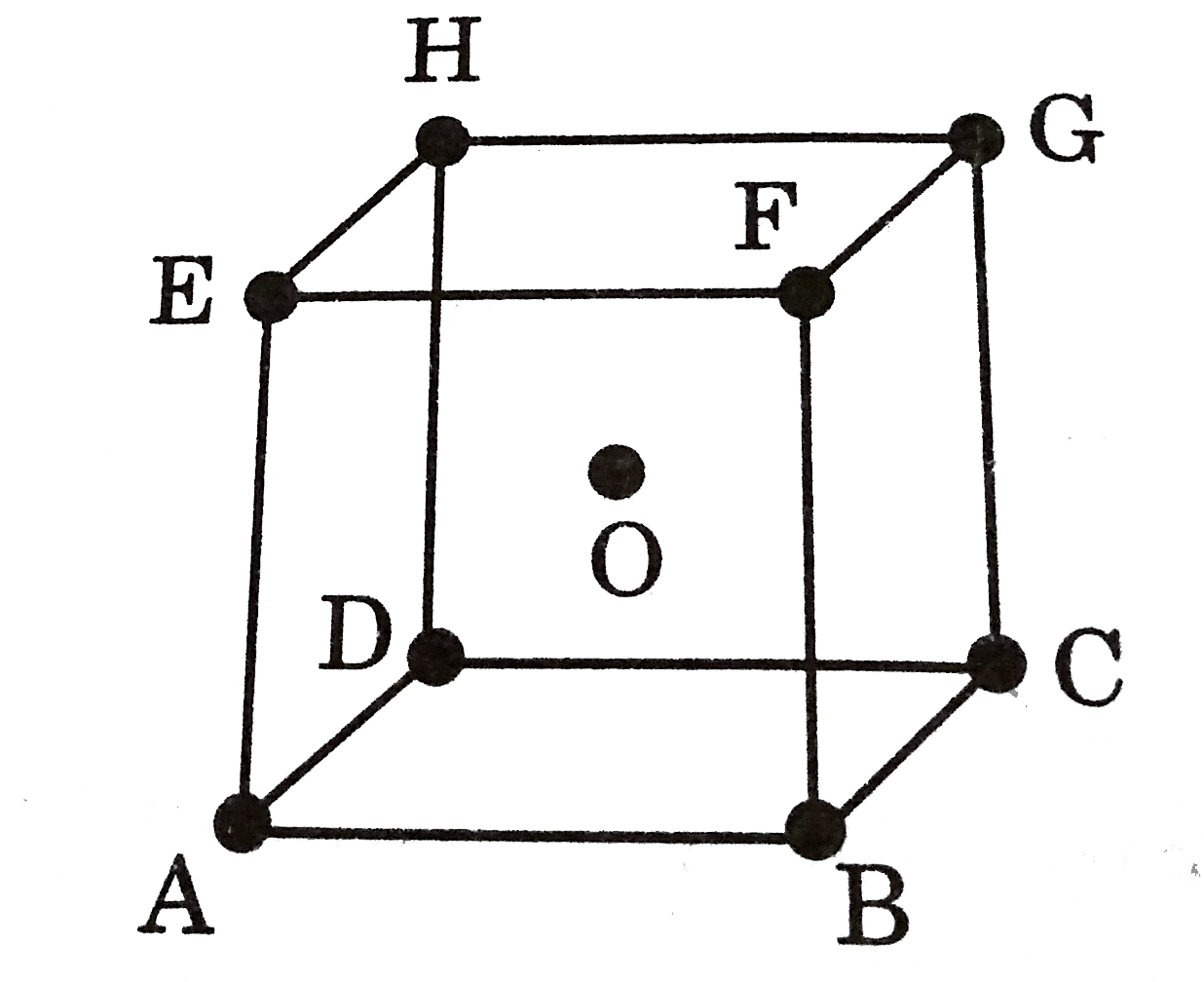
O is the body center, A, B, C, D, …,H are the corners. What is the magnitude of the angle AOB?
View Solution
Knowledge Check
In a body centred cubic arrangement, the number of atoms per unit cell is-
A8B2C1D4In a body centred cubic cell an atom at the body of centre is shared by-
A1 unit cellB4 unit cellsC3 unit cellsD2 unit cellsIn a body centred cubic cell an atom at the body of centre is shared by-
A1 unit cellB4 unit cellC2 unit cellD8 unit cell
In Body centered cubic structures the atoms are not present at?
View SolutionIn a body centred cubic structure, the space occupied is about
View SolutionIn a body-centred cubic arrangement the ion A occupies the centre while the ions of B occupy the corners of a cube. The formula of the crystal is:
View SolutionIn body centred cubic structure the space occupied is about
View SolutionSodium crystallises in bcc arrangement with the interfacial separation between the atoms at the edge length of 53 pm. The density of the solid is
View Solution

