(i) What is a π-bond?
(ii)Discuss the formation of N2 molecule using MO theory.
(i) Pi-Bond: When two atomic orbitals overlap sideways, the resultaint covalet bond is called a pi (π) bond.
(ii) Formation of N, molecule using MO theory:
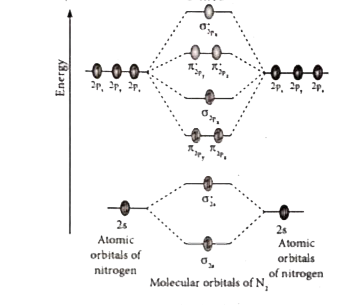
1. Electronic configuration of N atom 1s22s22p3 .
2. Electronic confifuration of N2 molecule is σ1s2σ∗1s2σ2s2σ∗2s2π2p2yπ2p2zσ2p2x
3. Bond order Nb−(Na)210−42=3
4. Molecule has no unpaired electrones hence, it is diamagnetic.
Topper's Solved these Questions
- SOLVED PAPER 03Book:FULL MARKSChapter:SOLVED PAPER 03Exercise:PART-III
Explore 9 Videos
- SOLUTIONS Book:FULL MARKSChapter:SOLUTIONS Exercise:ADDITIONAL QUESTIONS SOLVED - NUMERICAL PROBLEMS
Explore 15 Videos
- SOLVED PAPER 13 (UNSOLVED)Book:FULL MARKSChapter:SOLVED PAPER 13 (UNSOLVED)Exercise:PART-II
Explore 9 Videos
Similar Questions
Distinguish between oxidation and reaction.
View SolutionCalculate the mole fraction of benzene is solution containing 30% by mass in carbon tetrachloride.
View SolutionDefine - equivalent mass.
View Solutionसंयोजकता आबन्ध सिद्धान्त के आधार पर H2 अणु के विरचन (निर्माण) कि व्याख्या कीजिए ।
View SolutionThe number of electrons involved in the bond formation of N2 molecule are ________.
View SolutionThe ratio of number of σ-bond to π-bond in N2 and CO molecules are :
View SolutionDiscuss the formation of N2 molecule using MO theory
View SolutionExplain the formation H2 molecule on the basis of Valence Bond theory.
View SolutionDiscuss the formation of N2, molecule using MO Theory.
View Solution(i) Write the resonance structures for ozone molecule and N_(2)O ? (ii) Draw MO diagram of CO and calculate its bond order.
View Solution" If the formation of diatomic boron molecule is predicted based on MO theory,the bond order would be "
View SolutionThe bond order of CO molecule on the basis of MO theory is
View SolutionIf the formation of diatomic boron molecule is predicted based on MO theory, the bond order would be
View SolutionExplain the formation of covalent bonds in nitrogen molecule (N_(2)) .
View SolutionThe number of electrons involved in the bond formation of N_(2) molecule are ________.
View Solution

