(i) CuCl is more covalent than NaCl. Give reason.
(ii) Draw and explain the molecular orbital diagram of Boron molecule.
Text Solution
(i) 1. Cations having ns2np6nd10 configuration show greater polarising power than the cations with ns2np6 configuration. Hence they show greater covalent character.
2. CuCl is more covalent than NaCl. As compared to Na+(1.13Å),Cu+(0.6Å) is small and has 3s23p63d10 configuration.
3. Electronic configuration of Cu+ : [Ar]3s23p63d10
electronic configuration of Na+ : [He]2s22p6
So CuCl is more covalent that NaCl
(ii) 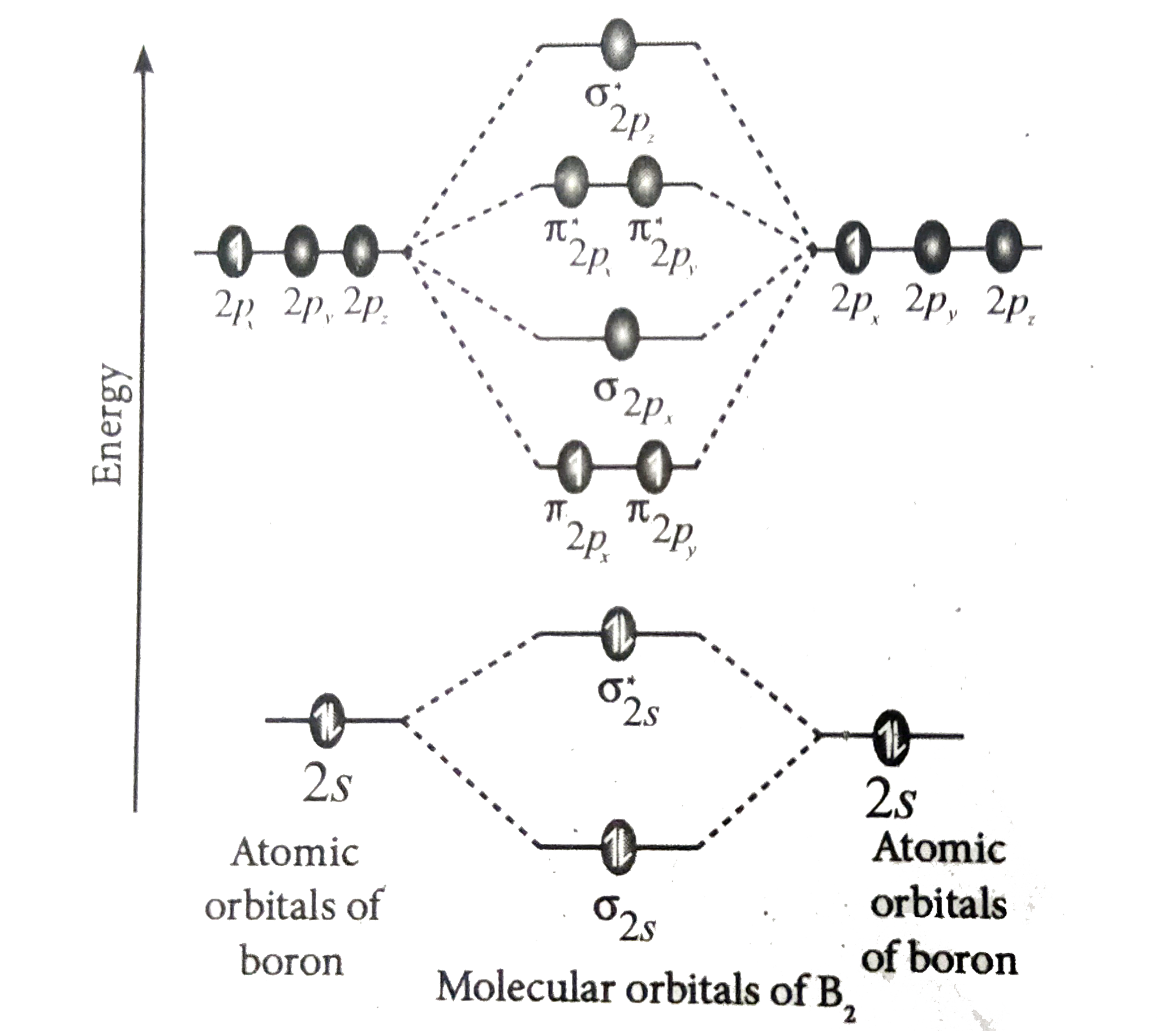
1. Electronic configuration of B=1s22s22p3
2. Electronic configuration of B2=σ1s2σ∗1s2σ2s2σ∗2s2π2p1xπ2p1z
3. Bond order =Nb−Na2=6−42=1
4. B2 molecule has two unpaired electrons hence it is paramagnetic.
|
Similar Questions
Knowledge Check
Assertion (A): CuCI is more covalent than NaCI.
Reason (R ): Na⊕ ion more polarising than Cu⊕ ion.AStatement-1 is True, Statement-2 is True, Statement-2 is a correct explanation for statement-1.BStatement-1 is True, Statement-2 is True, Statement-2 is not a correct explanation for statement-1.CStatement-1 is True, Statement-2 is False.DStatement-1 is False, Statement-2 is True.
Draw molecular orbital diagram for N+2 molecule.
View SolutionDraw molecular orbital energy level diagram for nitrogen molecule.
View SolutionExplain on the basis of molecular orbital diagram why O2 should be paramagnetic.
View SolutionThe correct molecular orbital diagram for F2 molecule in the ground state is
View SolutionWhy N2 is more stable than O2 ? Explain on the basis of molecular orbital theory .
View SolutionGive the molecular orbital description of hydrogen molecule and deduce the bond order.
View SolutionWith the help of molecular orbital theory, draw the molecular orbital energy level diagram for N2 molecule. Also calculate the bond order and predict the magnetic behaviour.
View Solution

