An organic compound (A) of a molecular formula C6H6 which simple aromatic hydrocarbon. A undergoes hydrogenation to give a cyclic compound (B). A reacts with chlorine in the presence of UV-light to give C which is used insecticide Indentify A,B and C. Explain the reactions with equation.
1. Simple aromatic hydrocarbon, C6H6 is benzene.
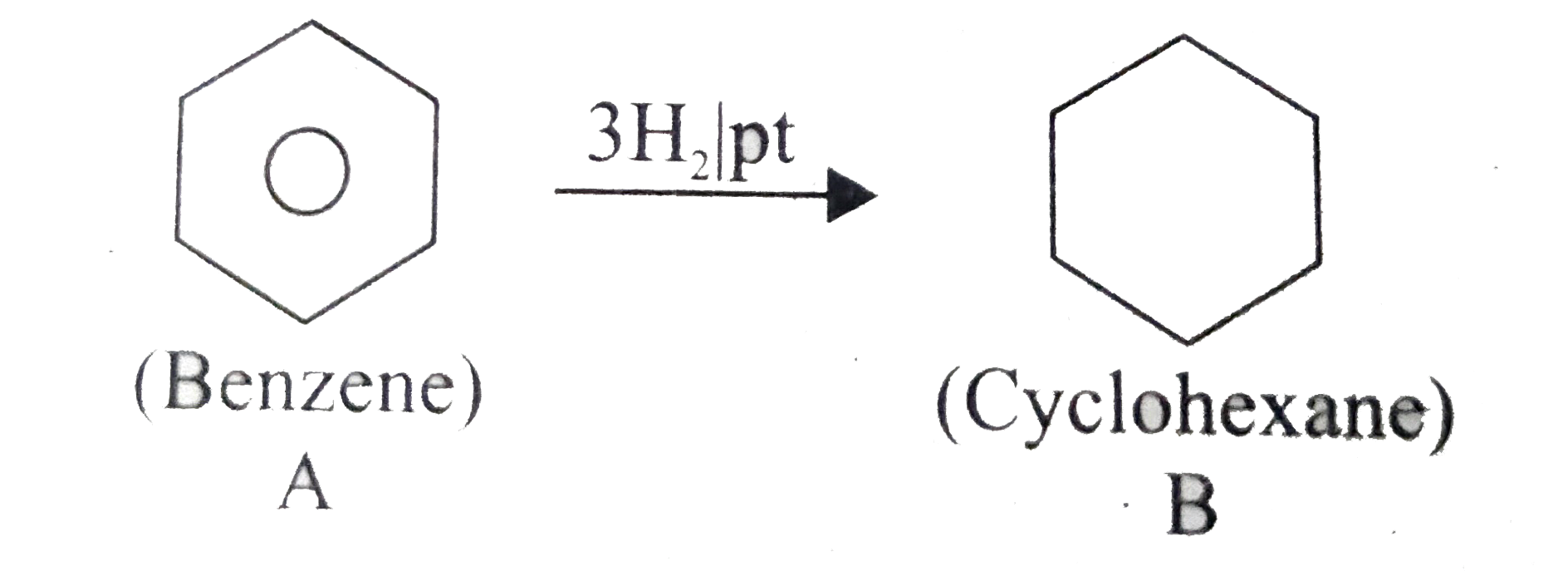
2. Benzene (A) reacts with H2 in the presence of Pt to give cyclohexane (B)
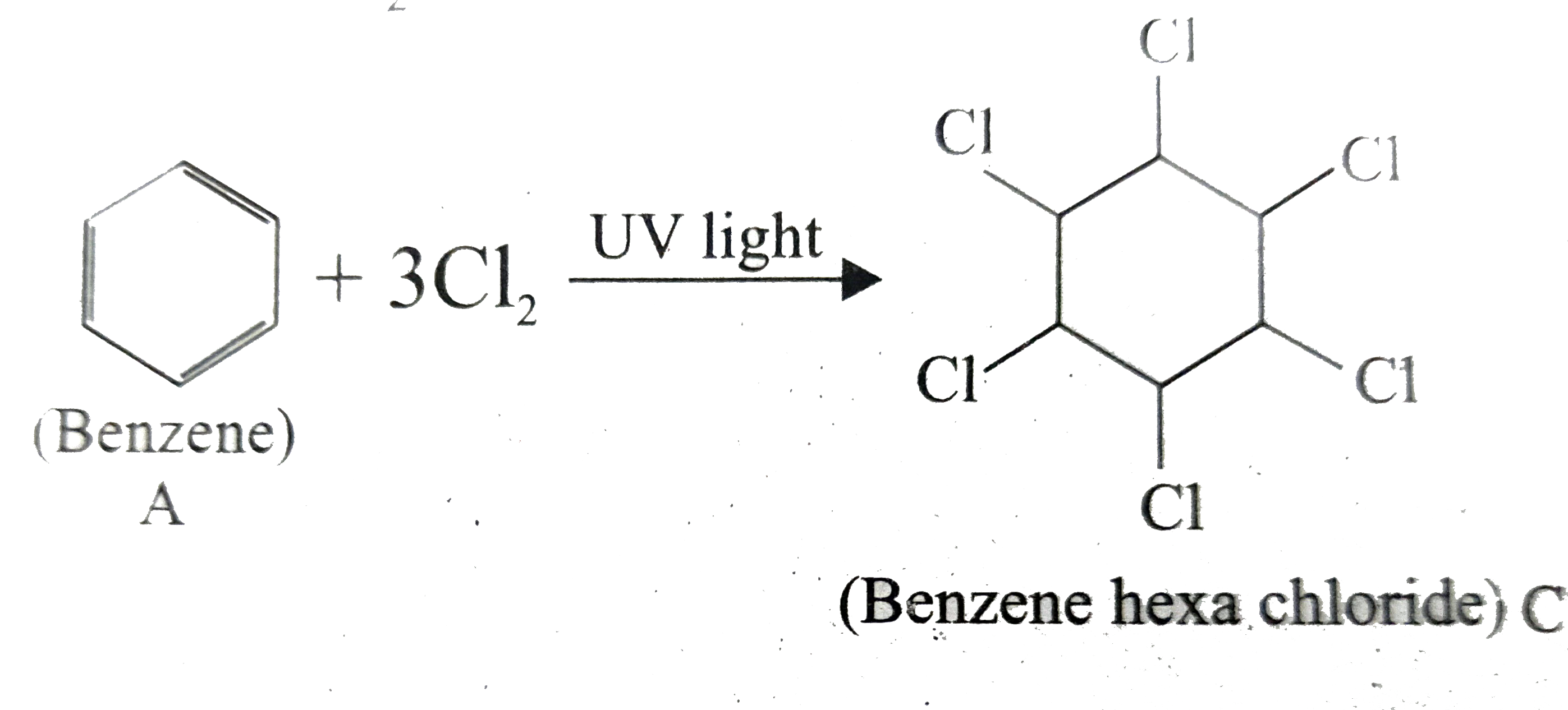
3. Benzene (A) reacts with Cl2 in presence of UV-light to give benzene hexachloride (C).
Similar Questions
(i) An organic compound (A) of molecular formula C2H6O7 on heating with conc.H2SO4 gives compound (B). (B) on treating with cold dilute alkaline KMnO4 gives compound (C). Identify (A). (B) and (C) and explain the reactions.
(ii) A simple aromatic hydrocarbon (A) reacts with chlorine to give compound (B). Compound (B) reacts with ammonia to give compound (C) which undergoes carbylamine reaction. Identify (A), (B) and (C) and explain the reactions.
View Solution(b) A aromatic hydrocarbon (A) of molecular formula C_(6)H_(6) reacts with Conc. HNO_(3) and Conc. H_(2)SO_(4) gives (B) of formula C_(6)H_(5)O_(2)N . (B) on reaction with Sn//HCl gives (C ) of formula C_(6)H_(7)N which answers carbylamine reaction. (C ) on treatment with chloroform and alkali gives (D ) of formula C_(7)H_(5)N . Identify A, B, C, D and explain the reactions involved.
View SolutionAn organic compound (A) of molecular formula C6H5Cl on boiling with hot water gives (B) of molecular formula C6H6O. (B) on reaction with Zinc dust gives (C) a simplest aromatic hydrocarbon. (C) on reaction with methyl chloride in the presence of anhydrous AlCl2 gives (D) of molecular formula C7H8. ldentify A, B, C, D and explain the reaction.
View SolutionAn organic compound (A) of molecular formula C_2H_6O on reaction with acidified K_2Cr_2O_7 gives (B) of molecular formula C_2H_4O which on further oxidation gives (C) of molecular formula C_2H_4O . Compound (C) reacts with (A) in the presence of conc. H_2SO_4 gives (D) of molecular formula C_4H_8O_2 . Identify A,B,C,D and explain the reaction involved.
View SolutionA aromatic hydrocarbon (A) of molecular formula C_(6)H_(6) reacts with Con HNO_(3) and Conc. H_(2)SO_(4) gives (B) of formula C_(6)H_(5)O_(2)N . (B) on reaction with Sn/HCl gives (C) formula C_(6)H_(7)N which answers carbylamine reaction. (C) on treatment with chloroform and alkalli gives (D) of formula C_(7)H_(5)N . Identify A,B,C,D and explain the reactions involved.
View SolutionA Compound (A) with molecular formula C2H3N on acid hydrolysis gives (B) which reacts with thionylchloride to give compound ( C ). Beczene reacts with compounds ( C ) in presence of anhydros AlCl3 to give compound ( C ). Compound ( C ) on reduction with gives (D). Identify (A), (B), (C) and D. Write the equations.
View SolutionAn organic compound A of molecular formula C_(2)H_(4) , on reduction gives another compound B of molecular formula C_(2) H_(6) .B on reaction with chlorine in the presence of sunlight gives C of molecular formula C_(2)H_(5) CI . The compounds A, B and Care:
View Solution

