Describe the Aufbau principle.
Text Solution
In the ground state of the atoms,the orbitals are in the order of their increasing energies.
That is the electrons first occupy the lowest energy orbitals.
The order of filling of various orbitals as per Aufbau principle is 1slt2slt2plt3slt3plt4slt3dlt4plt5slt4d..........
For e.g.,K(Z=19)
The electronic configuration is 1s22s22p63s23p64s1.
After filling 4s orbital only we have to fill up 3d orbital.
|
Topper's Solved these Questions
- QUANTUM MECHANICAL MODEL OF ATOMBook:FULL MARKSChapter:QUANTUM MECHANICAL MODEL OF ATOMExercise:IN-TEXT QUESTION-EVALUATE YOURSELF
Explore 8 Videos
- QUANTUM MECHANICAL MODEL OF ATOMBook:FULL MARKSChapter:QUANTUM MECHANICAL MODEL OF ATOMExercise:ADDITIONAL QUESTIONS SOLVED (choose the Correct Answer)
Explore 11 Videos
- QUANTUM MECHANICAL MODEL OF ATOMBook:FULL MARKSChapter:QUANTUM MECHANICAL MODEL OF ATOMExercise:Additional Questions Solved (5-Mark Questions)
Explore 6 Videos
- PHYSICAL AND CHEMICAL EQUILIBRIUMBook:FULL MARKSChapter:PHYSICAL AND CHEMICAL EQUILIBRIUMExercise:ADDITIONAL QUESTIONS SOLVED (NUMERICAL PROBLEMS)
Explore 3 Videos
- SAMPLE PAPER - 10 (SOLVED)Book:FULL MARKSChapter:SAMPLE PAPER - 10 (SOLVED)Exercise:PART - IV
Explore 10 Videos
Similar Questions
Knowledge Check
the current statement on the Aufbau principle is that
A(n-1)d subshell is always lower in energy than ns orbitalB(n-1)f subshell always has energy more than np subshellC5d is lower in energy than 4fD6p is lower in energy than 5dAssertion: La should be the f-block element according to Aufbau principle.
AIf both (A) and (R) are correct and (R) is the correct explanation of (A).BIf both (A) and (R) are correct, but (R) is not the correct explanation of (A).CIf (A) is correct, but (R) is incorrect.DIf (A) is incorrect but (R) is correct.Which is not in accordance to Aufbau principle
A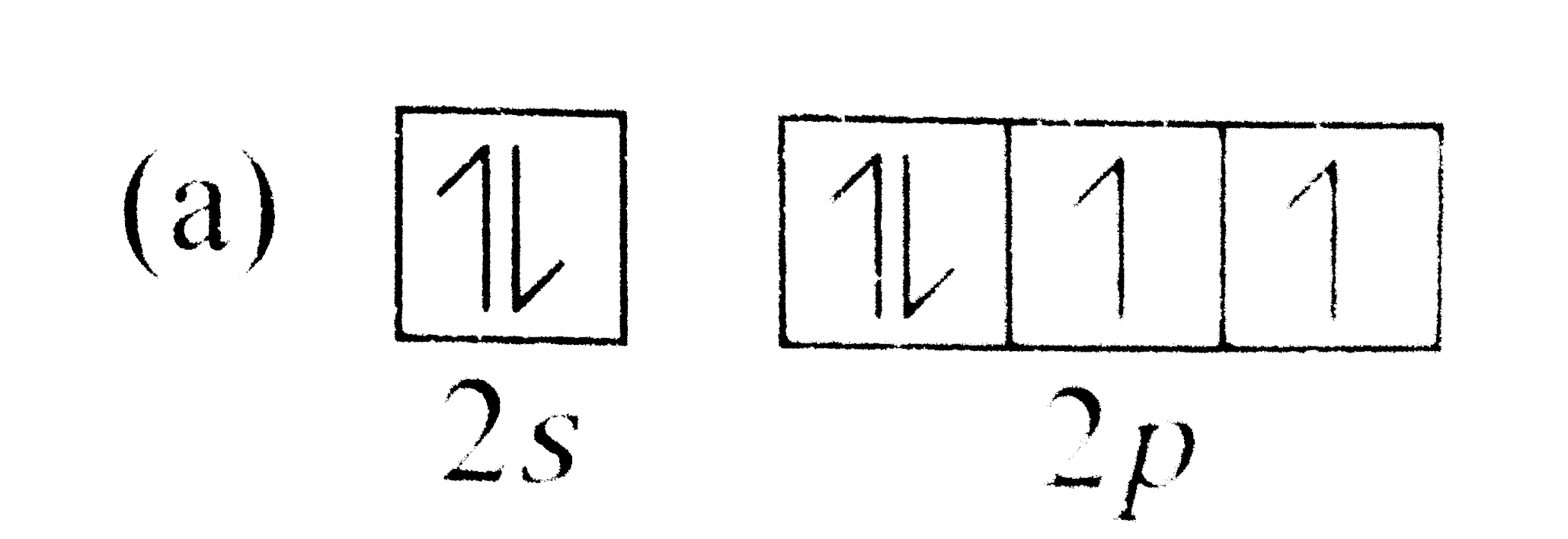 B
B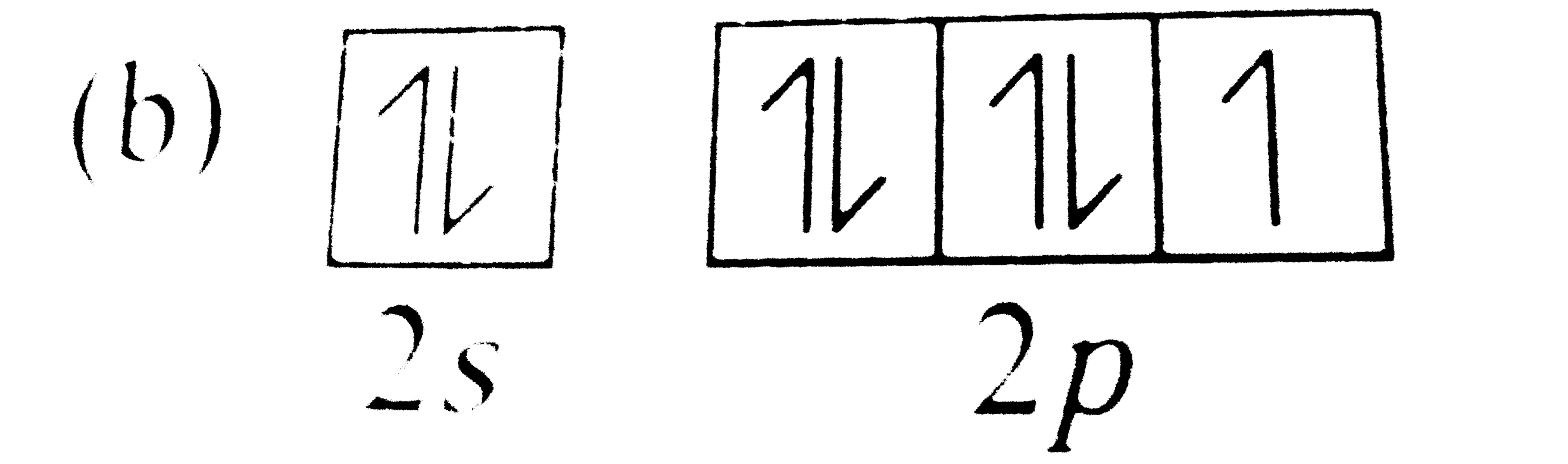 C
C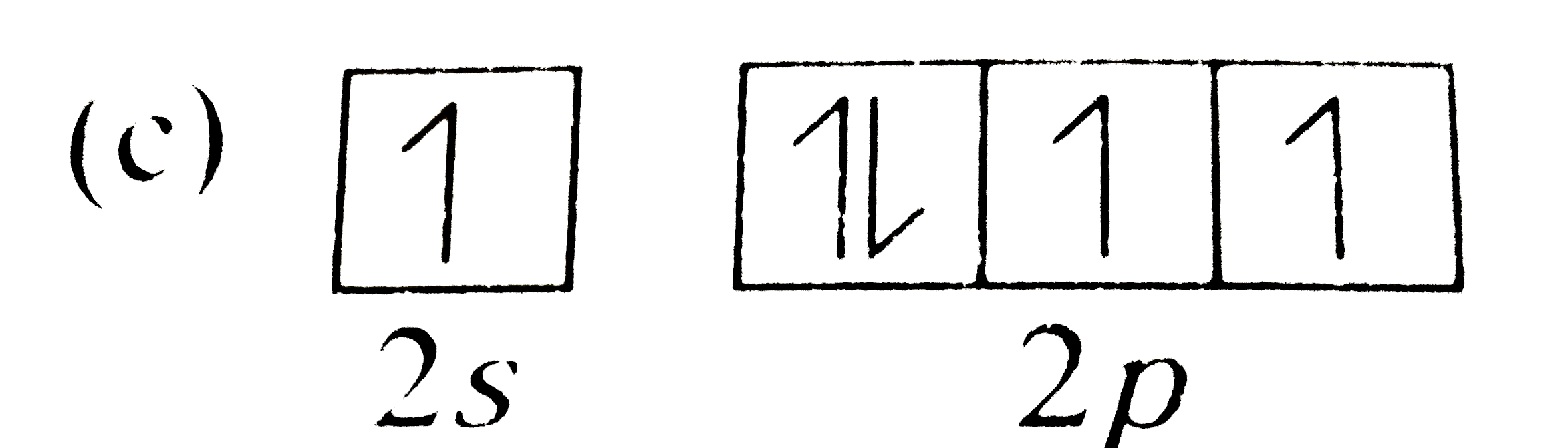 D
D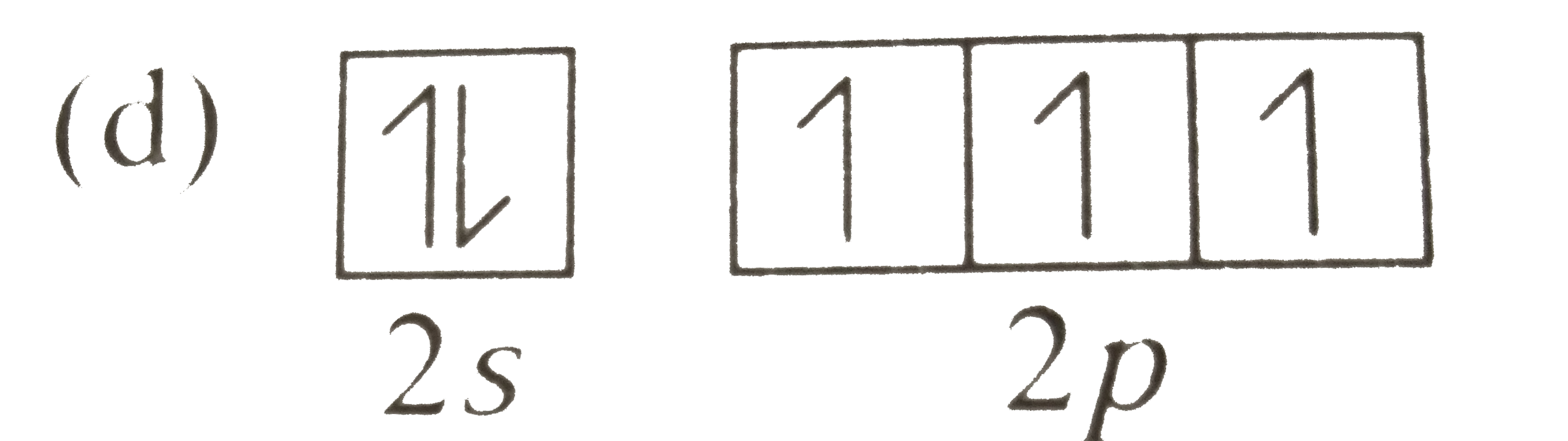
Write short notes on
(i) Pauli's exclusion principle
(ii) Aufbau principle.
View SolutionThe orbital diagram in which the Aufbau principle is violated is
View SolutionThe orbital diagram in which the Aufbau principle is violated
View SolutionThe orbital diagram in which the Aufbau principle is violated is
View SolutionThe orbital diagram in which the Aufbau principle is violated
View Solution

