The value of cell emf of Mercury button cell is …………… .
A
1.35V
B
−0.76V
C
0.34V
D
100V
Text Solution
The correct Answer is:A
|
Similar Questions
Knowledge Check
The EMF of a cell is
Asum of two oxidation potentialsBsum of two potentialsCdifference of two electrode potentialsDNone of the aboveThe electrolyte used in mercury cell is
Aaqueous copper sulphate solutionBaqueous zinc sulphate solutionCpaste of mercuric oxide mixed with KOHDdilute sulphuric acidAssertion: The cell potential of mercury cell is 1.35V which remains constant.
Reason: In mercury cell, the electrolyte is a paste of KOH and ZnO.AIf both assertion and reason are true, and reason is the true explanation of the assertionBif both assertion and reason are true, but reason is not the true explanation of the assertion.Cif assertion is true, but reason is false.DIf both assertion and reason are false.
Write the working of mercury dry cell
View SolutionWhat is the cell potential of mercury cell ? What are its applications ?
View SolutionFrom the given cells :
Lead storage cell , Mercury cell , Fuel cell and Dry cell
Answer the question
Which cell is used in hearing aids ?
View SolutionAssertion: The cell potential of mercury cell is 1.35V which remains constant.
Reason: In mercury cell, the electrolyte is a paste of KOH and ZnO.
View SolutionThe two half cell reactions of an electrochemical cell is given as
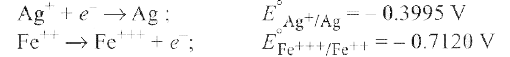
the value of cell EMF will be
View Solution

