Explain about the variation of molar conductivity with concentration by Kohlraush studies?
Text Solution
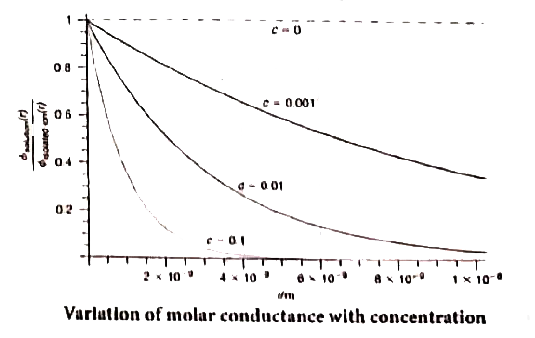
Kohlraush observed that, increase of molar conductance of an electrolytic solution with the increase in dilution. He deduced the following empirical relationship betwee the molar conductance (Λm) and concentration of the electrolyte (c )
Λm=Λ∘m−k√c
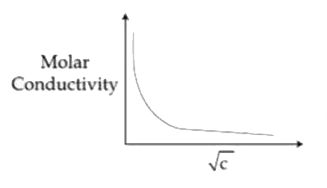
For strong electrolytes such as KCl,NaCl the plot ΛmVs√c, gives a straight line. It is also observed that the plot is not a linear one for weak electrolyte.
|
Topper's Solved these Questions
- ELECTRO CHEMISTRYBook:FULL MARKSChapter:ELECTRO CHEMISTRYExercise:ADDITIONAL QUESTIONS -5- MARK QUESTIONS
Explore 15 Videos
- ELECTRO CHEMISTRYBook:FULL MARKSChapter:ELECTRO CHEMISTRYExercise:ADDITIONAL QUESTIONS -2- MARK QUESTIONS
Explore 20 Videos
- COORDINATION CHEMISTRYBook:FULL MARKSChapter:COORDINATION CHEMISTRYExercise:Additional Question(5 Marks Question)
Explore 26 Videos
- HYDROXY COMPOUNDS AND ETHERSBook:FULL MARKSChapter:HYDROXY COMPOUNDS AND ETHERSExercise:ADDITIONAL QUESTIONS (Answer the following)
Explore 115 Videos
Similar Questions
Knowledge Check
Wrong about molar conductivity is
Athe solution contains Avogadro's number of molecules of the electrolyteBit is the product of speciific conductivity and volume of solution in cc containing 1 mole of the electrolyteCits units are ohm−1cm2mol−1Dit value for 1 M NaCl solution is same as that of 1 M glucose solutionFor a dilute solution of a strong electrolyte, the variation of molar conductivity with concentration is given by
A∧m=∧∘.m+AcB∧m=∧∘.m−AcC∧m=∧∘.m+A√cD∧m=∧∘.m−A√cWhich of the following plots represents correctly the variation of conductivity with concentration?
A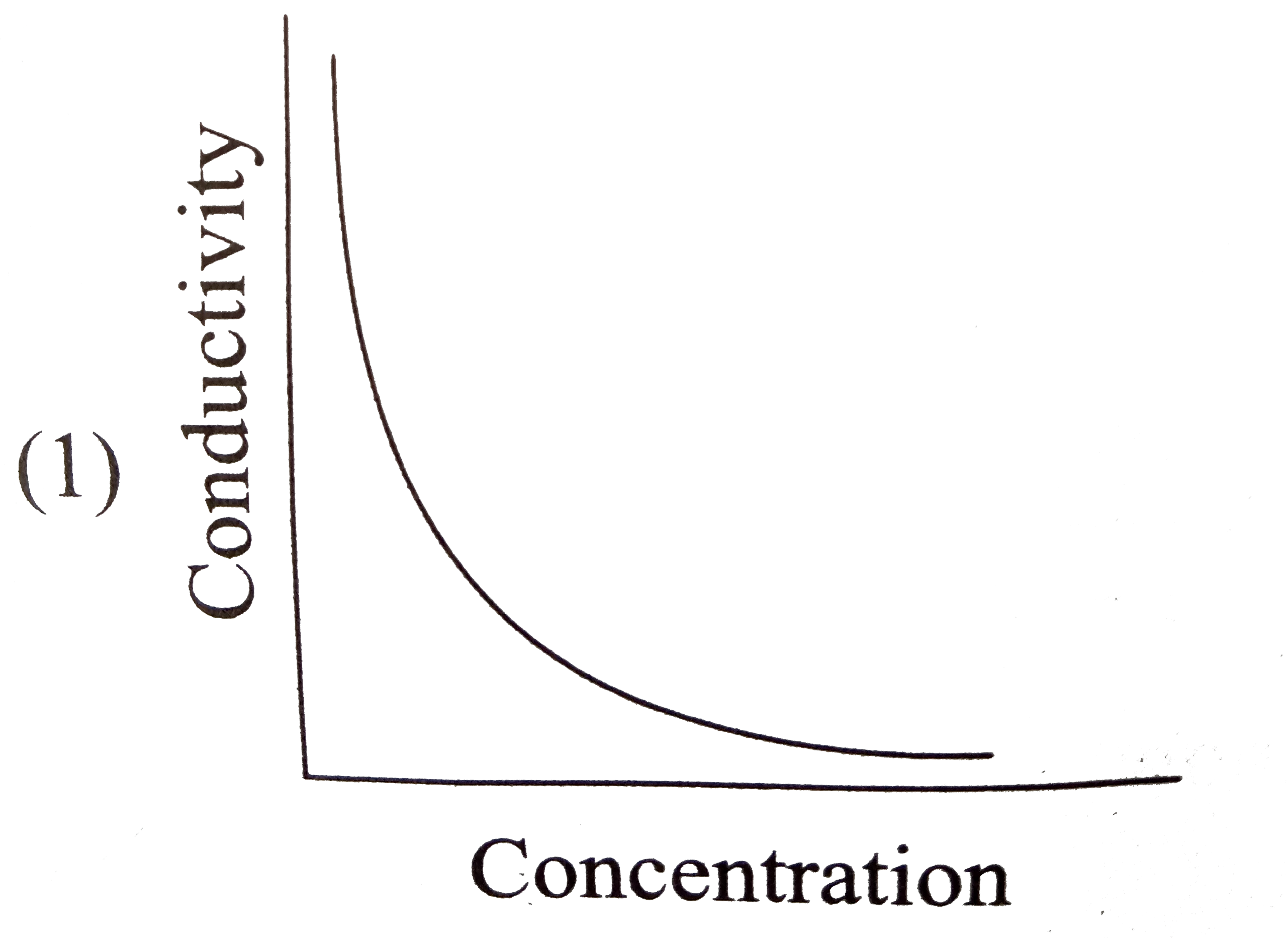 B
B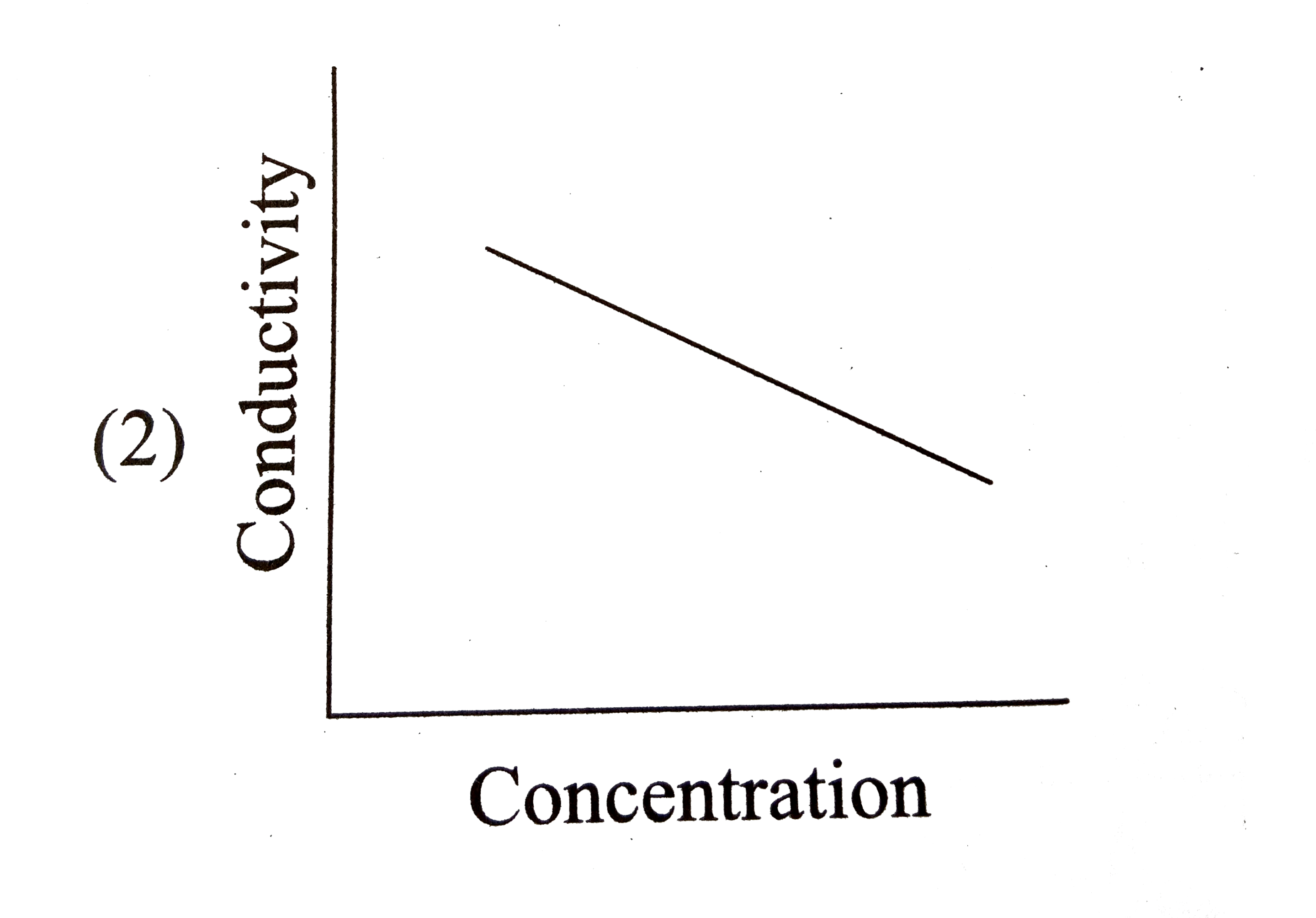 C
C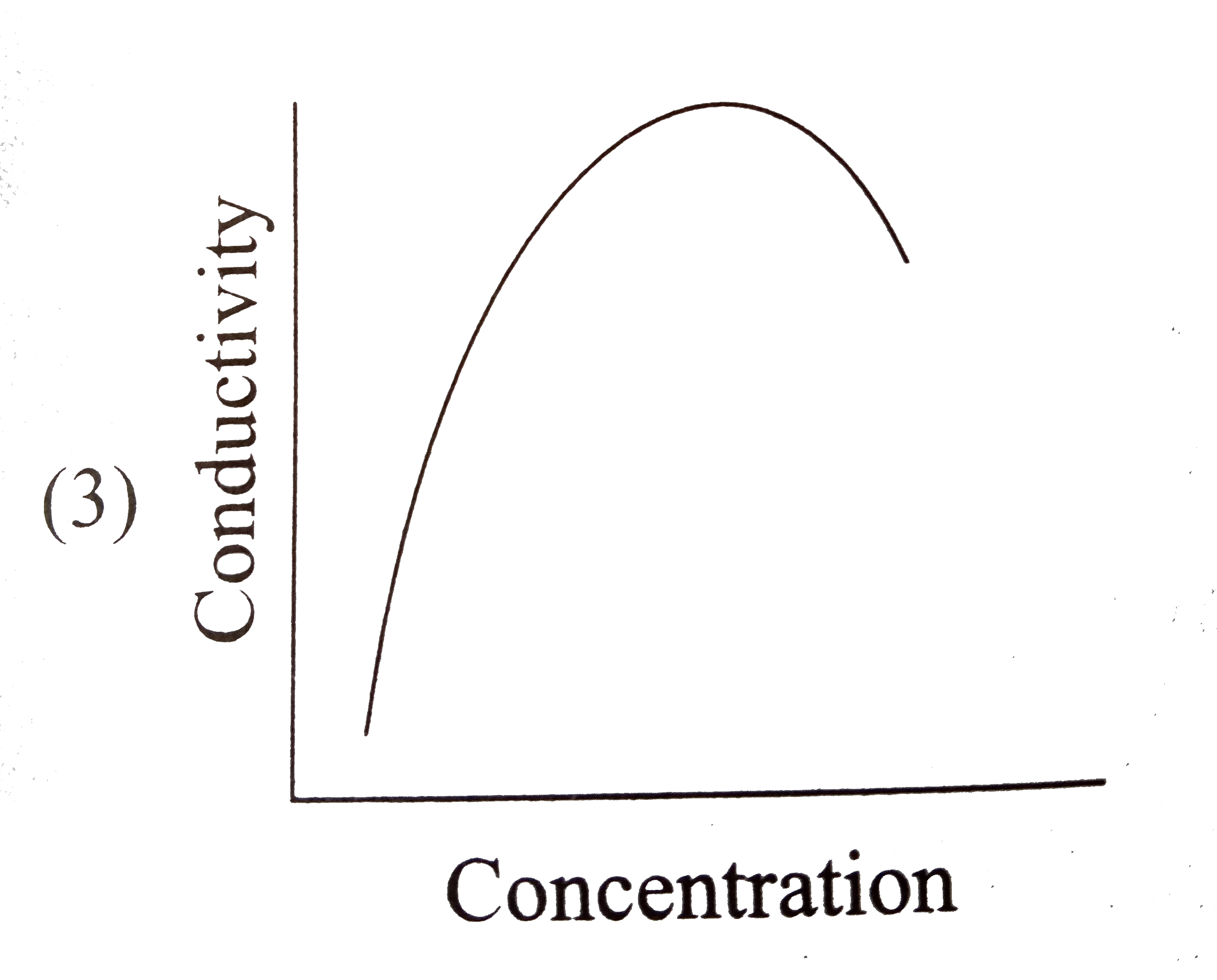 D
D
For a dilute solution of a strong electrolyte, the variation of molar conductivity with concentration is given by
View SolutionThe variation of molar conductivity wih concentration of an electrolyte (X) in aqueous solution is shown in the given figure .
The electrolyte X is :
View SolutionThe variation of molar conductivity wih concentration of an electrolyte (X) in aqueous solution is shown in the given figure .

The electrolyte X is :
View SolutionExplain the variation of conductivity with temperature for
ionic conductors.
View SolutionThe variation of molar conductivity wih concentration of an electrolyte (X) in aqueous solution is shown in the given figure .

The electrolyte X is :
View Solution

