How will you calculate the reduction potential of Half cell?
(i) Consider the zinc electrode dipped in zinc sulphate solution using SHE
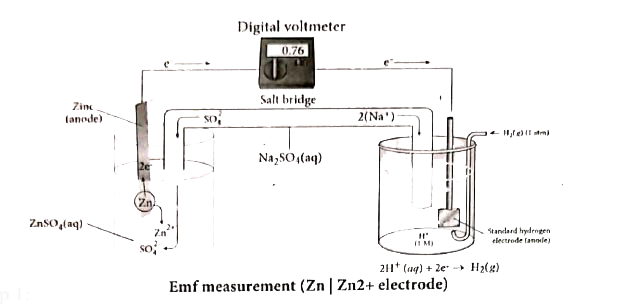
(ii) Step 1 :
The following galvanic cell is constructed using SHE
Zn(s)∣∣Zn2+(aq.1M)∣∣∣∣H+(aq.1M)∣∣H2(g,1atm)∣Pt(s)
(iii) Step 2:
The emf of the above galvanic cell is measured using a voltmeter. In this case the measured emf of the above galvanic cell is 0.76 V .
(iv) Calculation:
We know that,
E∘cell=(E∘ox)Zn/Zn2++(E∘red)SHE
∴E∘cell=0.76+0V
=0.76V
The oxidation potential corresponds to the below mentioned half cell reaction which takes place at the cathode.
Zn→Zn2++2e− (oxidation)
(v) The emf of the reverse reaction will give the reduction potential
Zn2++2e−→Zn (E∘cell)Zn2+∣Zn=−0.76V
Topper's Solved these Questions
- ELECTRO CHEMISTRYBook:FULL MARKSChapter:ELECTRO CHEMISTRYExercise:ADDITIONAL QUESTIONS -3- MARK QUESTIONS
Explore 22 Videos
- COORDINATION CHEMISTRYBook:FULL MARKSChapter:COORDINATION CHEMISTRYExercise:Additional Question(5 Marks Question)
Explore 26 Videos
- HYDROXY COMPOUNDS AND ETHERSBook:FULL MARKSChapter:HYDROXY COMPOUNDS AND ETHERSExercise:ADDITIONAL QUESTIONS (Answer the following)
Explore 115 Videos
Similar Questions
The standard reduction potential for the half cell :
NO−3(aq)+2H++e−→NO2(g)+H2O is 0.78V.
a. Calculate the reduction potential in 8MH⊕.
b. What will be the reduction potential of the half cell in a neutral solution ? Assume all the other species to be at unit concentration.
View SolutionThe standard reduction potential for the half cell :
NOc−3(aq)+2Hc−+e−→NO2(g)+H2O is 0.78V.
a. Calculate the reduction potential in 8MH⊕.
b. What will be the reduction potential of the half cell in a neutral solution ? Assume all the other species to be at unit concentration.
View Solution
Knowledge Check
The potential of the following cell is 0.34 V at 25∘C calculate the standard reduction potential of the copper half cell
Pt|H2(1atm)|H+(1M)∣∣∣Cu2+(1M)∣∣CuA−3.4VB+3.4VC−0.34VD+0.34VThe hydrogen electrode is dipped in a solution of pH 3 at 25∘ C. The reduction potential of half cell would be :
A0.177VB−0.177VC0.087VD0.059V
The standard reduction potential for the half-cell, NO−3(aq.)+2H+(aq.)+e−→NO2(g)+2H2O is 0.78V.
(i) Calculate the reduction potential in 8M H+.
(ii) What will be the reduction potential of the half-cell in a neutral solution. Assume all the other species to be at unit concentration
View SolutionDerive expression to calculate emf (reduction) of the following half cells at 25∘C
Fe3+∣Fe2+(Pt)
View SolutionDerive expression to calculate emf (reduction) of the following half cells at 25∘C
AgCl(s),Cl−∣Ag
View SolutionDerive expression to calculate emf (reduction) of the following half cells at 25∘C
H+∣∣H2(Pt)(1atm)
View SolutionDerive expression to calculate emf (reduction) of the following half cells at 25∘C
Cu2+∣Cu(s)
View SolutionHow the reduction potential of an electrode can be decreased?
View Solution

