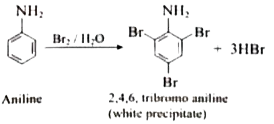
Explain the action of Br2 water with aniline.
Text Solution
|
Topper's Solved these Questions
- ORGANIC NITROGEN COMPOUNDS Book:FULL MARKSChapter:ORGANIC NITROGEN COMPOUNDS Exercise:ADDITIONAL QUESTIONS ( 3- MARK QUESTIONS)
Explore 40 Videos
- ORGANIC NITROGEN COMPOUNDS Book:FULL MARKSChapter:ORGANIC NITROGEN COMPOUNDS Exercise:ADDITIONAL QUESTIONS (5-MARK QUESTIONS)
Explore 14 Videos
- ORGANIC NITROGEN COMPOUNDS Book:FULL MARKSChapter:ORGANIC NITROGEN COMPOUNDS Exercise:ADDITIONAL QUESTIONS (Find the odd one out give the reasons )
Explore 6 Videos
- METALLURGY Book:FULL MARKSChapter:METALLURGY Exercise:Additional questions - ( 5 MARK QUESTIONS )-Answer the following questions.
Explore 8 Videos
- P - BLOCK ELEMENTS - IIBook:FULL MARKSChapter:P - BLOCK ELEMENTS - IIExercise:Additional Questions (5 Mark Questions)
Explore 11 Videos
Similar Questions
Knowledge Check
Glucose on reaction with Br2 water gives
Aglucaric acidBgluconic acidCsaccharic acidDcitric acidThe reaction of ethylene with Br2 in water in the presence of NaCl gives
A1,2-dibromoethaneB1-bromo-2-chloroethaneC2-bromoethanolDAll of theseThe reaction of ethylene with Br2 in water in the presence of NaCI gives:
A1,2-dibromoethaneB2-bromoethanolC1-bromo-2-chloroethaneDall of these
Glucose on reaction with Br2 water gives
View SolutionEthylamine is soluble in water whereas aniline is not soluble in water.
View SolutionMethylamine is soluble in water but not aniline. Explain.
View SolutionThe reaction of ethylene with Br2 in water in the presence of NaCl gives :
View SolutionAssertion : Oxidation of glucose by Br2 water gives gluconic acid.
Reason : Br2 water oxidises both -CHO and -OH groups.
View Solution