Magnetic moments 2.84B.M is given by :
(At. nos. ni = 28, Ti = 22, Cr = 24, Co = 27).
A
Ti3−
B
Cr2+
C
Co2+
D
Ni2+
Video Solution
Text Solution
The correct Answer is:D
Magnetic moment =2.84 B.M . This indicates that 2 unpaired delectrons are present .
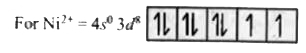
|
Similar Questions
Knowledge Check
Magnetic moments 2.84B.M is given by :
(At. nos. ni = 28, Ti = 22, Cr = 24, Co = 27).ACr2+BCo2+CNi2+DTi3+Magnetic moment 2.84 BM is given by
ACr2+BCo2+CNi2+DTi3+Magnetic moments 2.84B.M is given by :
(At. nos. ni = 28, Ti = 22, Cr = 24, Co = 27).ATi3−BCr2+CCo2+DNi2+
Magnetic moment 2.84B.M. is given by : (Atomic numbers. Ni=28, Ti=22, Cr=24, Co=27)
View SolutionMagnetic moments 2.84B.M is given by :
(At. nos. ni = 28, Ti = 22, Cr = 24, Co = 27).
View SolutionMagnetic moment 2.83 BM is given by which of the following ions?
At. nos. Ti=22, Cr=24, Mn=25,Ni=28
View SolutionThe magnetic moments of complexes given below are in the order: [Atomic no. Ti = 22 , Cr = 24 , Fe = 26 , Ni = 28 ]
[FeF6]3−
[Ti(H2O)6]3+
[Cr(NH3)6]3+
[Ni(H2O)6]2+
View SolutionWhich of the following paramagnetic ions would exhibit a magnetic moment (spin only) of the order of 5 BM?
(At. No : Mn = 25, Cr = 24,V = 23, Ti = 22)
View Solution
