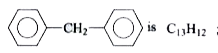
The molecular formula of diphenyl methane.

How many structural isomers are possible when one of the hydrogen is replaced by a chlorine atom?
Text Solution
The correct Answer is:4
In diphenylmethane monochlorination at following positions will produce structured isomers

|
Similar Questions
Knowledge Check
The molecular formula of diphenylmethane is

How many structural isomers are possible when one of the hydrogens is replaced by a chlorine atom ?A6B4C8D7The molecular formula of diphenylmethane,
How many structural isomers are possible when one of the hydrogen is replaced by a chlorine atom?A6B4C8D7The molecular formula of diphenylmethane,
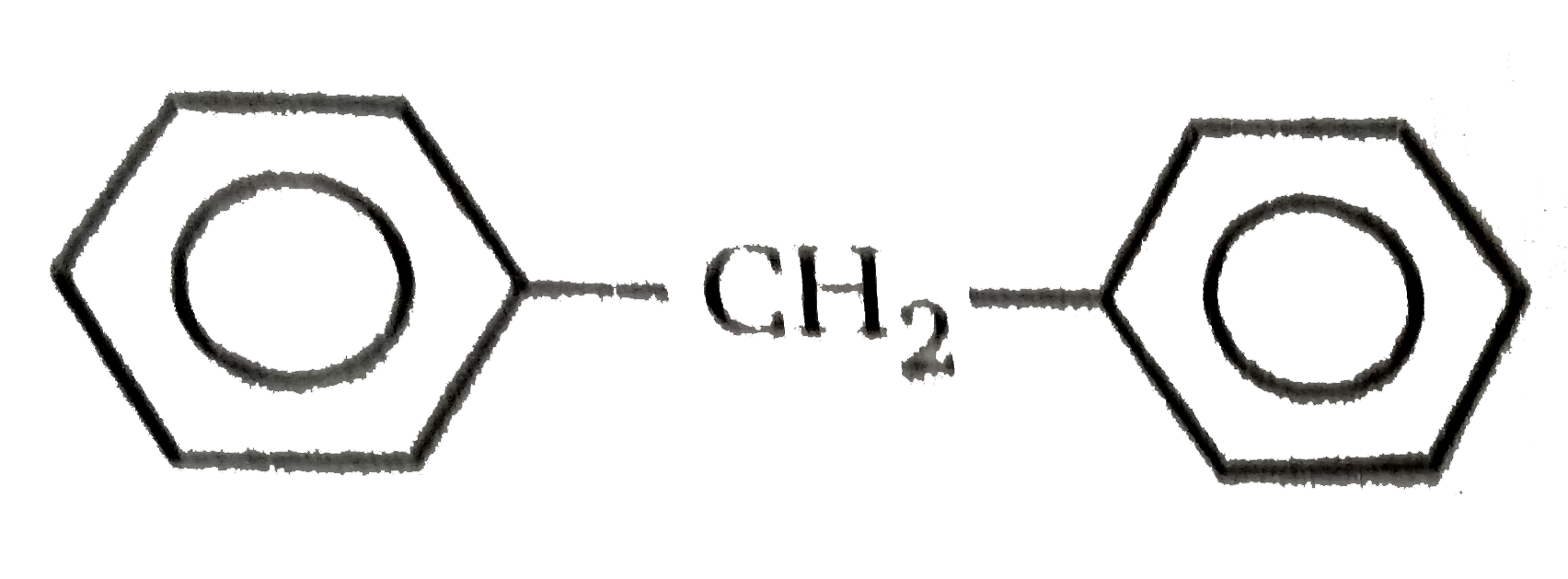
How many structural isomers are possible when one of the hydrogen is replaced by a chlorine atom ?A6B4C8D7
The molecular formula of dipheylmethane is C13H12
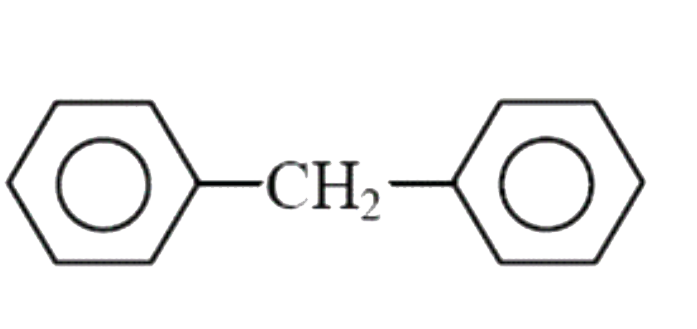
How many structural isomer are possible when one of the hydrogens is replaced by a chlorine atom?
View Solution
The molecular formula of diphenylmethane, How many structural isomers are possible when one of the hydrogen atoms is replaced by a chorine atom?
View SolutionHow many stuctural isomers are possible when one of the hydrogen is replaced by a chlorine atoms anthracene?
View SolutionHow many structural isomers are possible when one of the hydrogen is replaced by a chlorine atom in anthracene?
View SolutionThe molecule formula of diphenyl methane is C13H12.

How many structural isomers are possible when one of the hydrogen is replaced by a chlorine atom?
View Solution


