A small spherical monoatomic ideal gas bubble (γ=5/3) is trapped inside a liquid of density ρ (see figure). Assume that the bubble does not exchange any heat with the liquid. The bubble contains n moles of gas. The temperature of the gas when the bubble is at the bottom is T0, the height of the liquid is H and the atmospheric pressure P0 (Neglect surface tension).
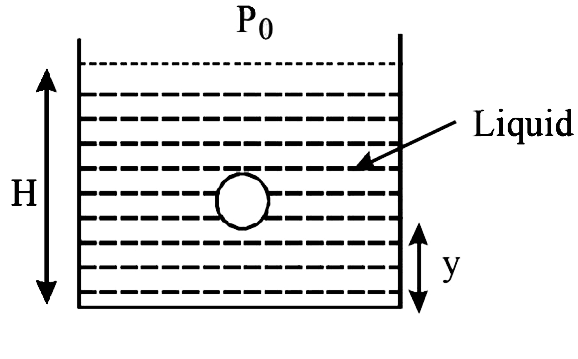
As the bubble moves upwards, besides the buoyancy force the following forces are acting on it
only the force of gravity
The force due to gravity and the force due to the pressure f the liquied
The force due to gravity the force due to the pressure of the liqueid and the force due to visocsity of the liquid
The force due to gravity and the force due to visosity of the liquid
The correct Answer is:D
Step by step video & image solution for A small spherical monoatomic ideal gas bubble (gamma=5//3) is trapped inside a liquid of density rho (see figure). Assume that the bubble does not exchange any heat with the liquid. The bubble contains n moles of gas. The temperature of the gas when the bubble is at the bottom is T_0, the height of the liquid is H and the atmospheric pressure P_0 (Neglect surface tension). As the bubble moves upwards, besides the buoyancy force the following forces are acting on it by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- JEE ADVANCEDBook:JEE ADVANCED PREVIOUS YEARChapter:JEE ADVANCEDExercise:MULTIPLE CHOICE QUESTIONS
Explore 22 Videos
- JEE ADVANCEDBook:JEE ADVANCED PREVIOUS YEARChapter:JEE ADVANCEDExercise:SECTION-III (Comprehension Type )
Explore 3 Videos
- JEE (ADVANCE) 2020Book:JEE ADVANCED PREVIOUS YEARChapter:JEE (ADVANCE) 2020Exercise:SECTION 3
Explore 6 Videos
- JEE ADVANCED 2020Book:JEE ADVANCED PREVIOUS YEARChapter:JEE ADVANCED 2020Exercise:SECTION-3
Explore 6 Videos
Similar Questions
A small air bubble of radius r in water is at depth h below the water surface. If P0 is the atmospheric pressure, ρ is the density of water and T is the surface tension of water, what is the pressure inside the bubble?
View SolutionA colloidal system in which gas bubbles are dispersed in a liquid is known as
View Solution
Knowledge Check
A small spherical monoatomic ideal gas bubble (γ=5/3) is trapped inside a liquid of density ρ (see figure). Assume that the bubble does not exchange any heat with the liquid. The bubble contains n moles of gas. The temperature of the gas when the bubble is at the bottom is T0, the height of the liquid is H and the atmospheric pressure P0 (Neglect surface tension).
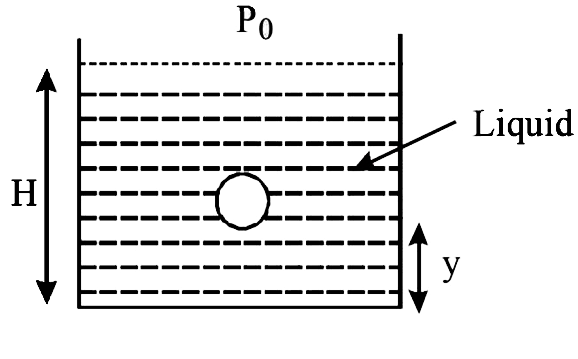
When the gas bubble is at a height y from the bottom, its temperature is-AT0(P0+ρlgHP0+ρlgy)2/5BT0(P0+ρlg(H−y)P0+ρlgH)2/5CT0(P0+ρlgHP0+ρlgy)3/5DT0(P0+ρlg(H−y)P0+ρlgH)3/5A small spherical monoatomic ideal gas bubble (γ=5/3) is trapped inside a liquid of density ρ (see figure). Assume that the bubble does not exchange any heat with the liquid. The bubble contains n moles of gas. The temperature of the gas when the bubble is at the bottom is T0, the height of the liquid is H and the atmospheric pressure P0 (Neglect surface tension).
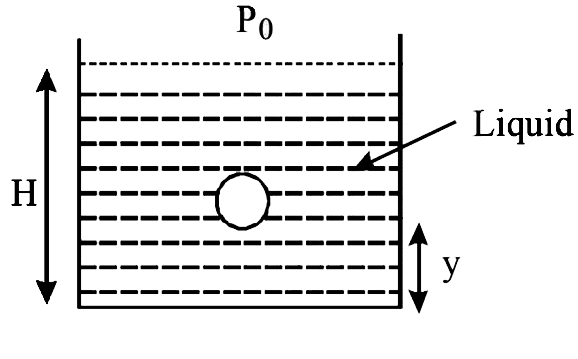
The buyoncy force acting on the gas bubble is (Assume R is the universal gas constant)AρlnRgT0(P0+ρlgH)2/5[P0+ρlgy]7/5BρlnRgT0(P0+ρlgH)2/5[P0ρlg(H−y)]3/5CρlnRgT0(P0+ρlgH)3/5[P0+ρlgy]8/5DρlnRgT0(P0+ρlgH)3/5[P0ρlg(H−y)]2/5A small spherical monoatomic ideal gas bubble (γ=53) is trapped inside a liquid of density ρl (see figure) . Assume that the bubble does not exchange any heat with the liquid. The bubble contains n moles of gas. The temperature of the gas when the bubble is at the bottom is T0, the height of the liquid is H and the atmospheric pressure is P0 (Neglect surface tension).
_E01_434_Q01.png)
When the gas bubble is at a height y from the bottom , its temperature is :-AT0(P0+ρlgHP0+ρlgy)2/5BT0(P0+ρlg(H−y)P0+ρlgH)2/5CT0(P0+ρlgHP0+ρlgy)3/5DT0(P0+ρlg(H−y)P0+ρlgH)3/5
At the boiling point , bubbles form within the liquid because
View SolutionAn air bubble is formed at depth h below the surface of water. The pressure inside the bubble is-
(P0= atmospheric pressure, r = radius of bubble)
View SolutionThe execc pressure inside the first soap bubble is three times that inside the second bubble. Then the ratio of the volumes of the first and second bubbles is
View SolutionThe gas inside a spherical bubble expands uniformly and slowly so that its radius increases from R to 2R. Let the atmospheric pressure be p0 and surface tension be S. The work done by the gas in the process is
View SolutionA small air bubble of radius r in water is at a depth h (r<<h). If Po is the atmospheric pressure, ρ is the density of water and T is the surface tension of water, the excess pressure inside the bubble over the pressure outside it is
View Solution
