In a periodic table, the average atomic mass of magnesium is given as 24.312u. The average value is based on their relative natural abundance on earth. The three isotopes and their masses are .12Mg24(23.98504u), .12Mg25(24.98584) and .12Mg26(25.98259u). The natural abundance of .12Mg24 is 78.99% by mass. Calculate the abundances of the other two isotopes. Solution in Tamil
2512Mg:9.3%,2612Mg:11.7%
Similar Questions
In a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their masses are 2412Mg(23.98504u),2512Mg(24.98584u)and2612Mg(25.98259u). The natural abundance of 2412Mg is 78.99% by mass. Calculate the abundances of other two isotopes.
View SolutionIn a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their masses are 2412Mg(23.98504u),2512Mg(24.98584u)and2612Mg(25.98259u). The natural abundance of 2412Mg is 78.99% by mass. Calculate the abundances of other two isotopes.
View SolutionIn a periodic table, the average atomic mass of magnesium is given as 24.312 u . The average value is based on their relative natural abundance on earth. The three isotopes and their masses are ._12Mg^(24) (23.98504u) , ._(12)Mg^(25) (24.98584) and ._12Mg^(26) (25.98259 u) . The natural abundance of ._12Mg^(24) is 78.99% by mass. Calculate the abundances of the other two isotopes.
View SolutionIn a periodic table, the average atomic mass of magnesium is given as 24,312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their masses are 2412Mg(23.98504 u), 2512Mg (24.98584 u) and 2612Mg(25.98259 u). The natural abundance of 2412Mg is 78.99% by mass. Calculate the abundances of the other two isotopes.
View SolutionIn the following table, seven elements P,Q,R,S,T,U and V (here letters are not the usual symbol of the elements)of the modern periodic table with their atomic numbers are given
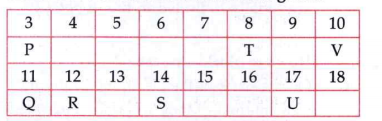 Which of these is an inert gas? Name it.
Which of these is an inert gas? Name it.
View SolutionAn element is found in nature in two isotopic forms with mass numbers (A-1) and (A+3). If the average atomic mass of the element is found to be A, then the relative abundance of the heavier isotope in the nature will be :
View SolutionIn a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their masses are 2412Mg (23.98504u), 2512Mg (24.98584u) and 2612Mg (25.98259u). The natural abundance of 2412Mg is 78.99% by mass. Calculate the abundances of other two isotopes.
View SolutionIn a periodic table the average atomic mass of magnesium is given as24.312u. The average value is based on their relative natural abundance on earth. The three isotopes and their masses are 2412Mg(23.98504u), 2512Mg(24.98584u)and 2612Mg(25.98259u). The natural abundance of 2412Mg is 78.99% by mass . Calculate the abundance of the other two isotopes.
View Solution
