The vapour pressure will be lowest for _______.
A
0.1 M sugar solution
B
0.1 M KCl solution
C
0.1MCu(NO3)2 solution
D
0.1MAgNO3 solution
Video Solution
Text Solution
The correct Answer is:C
Vapour pressire of a solvent is lowered by the presence of solute in it. Lowering in vapour pressure is a colligative property i.e., it depends on the no. of particles present in the solution. Cu(NO3)2 give the maximum no. of ions. (i.e., 3) so it causes the greatest lowering in vapour pressure of water.
|
Similar Questions
Knowledge Check
A temperature at which the vapour pressure of a solid is equal to the vapour pressure of liquid is called _______.
Aelevation of boiling pointBFreezing pointCboiling pointDdepression of freezing pointLowering of vapour pressure is highest for
A0.1 M BaCl2B0.1 M glucoseC0.1 M MgSo4DureaLowering of vapour pressure is highest for
AUreaB0.1 M glucoseC0.1MMgSO4D0.1MBaCl2


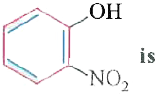 is higher than
is higher than 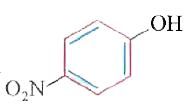 due to
due to