Which of the following has the highest value of radioactivity
1 g of Ra
1 g of RaSO4
1 g of RaBr2
1 g of Ra (HPO4)
The correct Answer is:A
To determine which of the given options has the highest value of radioactivity, we need to analyze the amount of radium present in each option, as radium is a radioactive element. The more radium present, the higher the radioactivity.
1. Identify the Options:
- Option 1: 1 gram of radium (Ra)
- Option 2: 1 gram of radium sulfate (RaSO₄)
- Option 3: 1 gram of radium bromide (RaBr₂)
- Option 4: 1 gram of radium hydrogen phosphate (Ra(H₂PO₄)₂)
2. Calculate the Radium Content in Each Option:
- Option 1:
- 1 gram of radium contains 100% radium.
- Option 2:
- Molar mass of radium sulfate (RaSO₄) = 226 (Ra) + 32 (S) + 4 × 16 (O) = 226 + 32 + 64 = 322 g/mol.
- Radium content in 1 gram of RaSO₄ = (226 g Ra / 322 g RaSO₄) × 1 g = 0.7 g Ra (approximately 70%).
- Option 3:
- Molar mass of radium bromide (RaBr₂) = 226 (Ra) + 2 × 80 (Br) = 226 + 160 = 386 g/mol.
- Radium content in 1 gram of RaBr₂ = (226 g Ra / 386 g RaBr₂) × 1 g = 0.585 g Ra (approximately 60%).
- Option 4:
- Molar mass of radium hydrogen phosphate (Ra(H₂PO₄)₂) = 226 (Ra) + 2 × 98 (H₂PO₄) = 226 + 196 = 422 g/mol.
- Radium content in 1 gram of Ra(H₂PO₄)₂ = (226 g Ra / 422 g Ra(H₂PO₄)₂) × 1 g = 0.535 g Ra (approximately 53%).
3. Compare the Radium Content:
- Option 1: 1 g Ra (100%)
- Option 2: 0.7 g Ra (70%)
- Option 3: 0.585 g Ra (approximately 60%)
- Option 4: 0.535 g Ra (approximately 53%)
4. Conclusion:
- The highest amount of radium is found in Option 1, which is pure radium (1 gram of radium). Therefore, Option 1 has the highest value of radioactivity.
Final Answer:
Option 1: 1 gram of radium has the highest value of radioactivity.
- Option 1: 1 gram of radium (Ra)
- Option 2: 1 gram of radium sulfate (RaSO₄)
- Option 3: 1 gram of radium bromide (RaBr₂)
- Option 4: 1 gram of radium hydrogen phosphate (Ra(H₂PO₄)₂)
- Option 1:
- 1 gram of radium contains 100% radium.
- Option 2:
- Molar mass of radium sulfate (RaSO₄) = 226 (Ra) + 32 (S) + 4 × 16 (O) = 226 + 32 + 64 = 322 g/mol.
- Radium content in 1 gram of RaSO₄ = (226 g Ra / 322 g RaSO₄) × 1 g = 0.7 g Ra (approximately 70%).
- Option 3:
- Molar mass of radium bromide (RaBr₂) = 226 (Ra) + 2 × 80 (Br) = 226 + 160 = 386 g/mol.
- Radium content in 1 gram of RaBr₂ = (226 g Ra / 386 g RaBr₂) × 1 g = 0.585 g Ra (approximately 60%).
- Option 4:
- Molar mass of radium hydrogen phosphate (Ra(H₂PO₄)₂) = 226 (Ra) + 2 × 98 (H₂PO₄) = 226 + 196 = 422 g/mol.
- Radium content in 1 gram of Ra(H₂PO₄)₂ = (226 g Ra / 422 g Ra(H₂PO₄)₂) × 1 g = 0.535 g Ra (approximately 53%).
- Option 1: 1 g Ra (100%)
- Option 2: 0.7 g Ra (70%)
- Option 3: 0.585 g Ra (approximately 60%)
- Option 4: 0.535 g Ra (approximately 53%)
- The highest amount of radium is found in Option 1, which is pure radium (1 gram of radium). Therefore, Option 1 has the highest value of radioactivity.
Option 1: 1 gram of radium has the highest value of radioactivity.
Similar Questions
Knowledge Check
Which of the following has the highest pH
A0.1 M NaOHB0.01 M NaOHC0.1 M HClD0.1MCH3COOHWhich of the following has the highest mp
APbBDiamondCFeDNaWhich of the following has highest Ka value ?
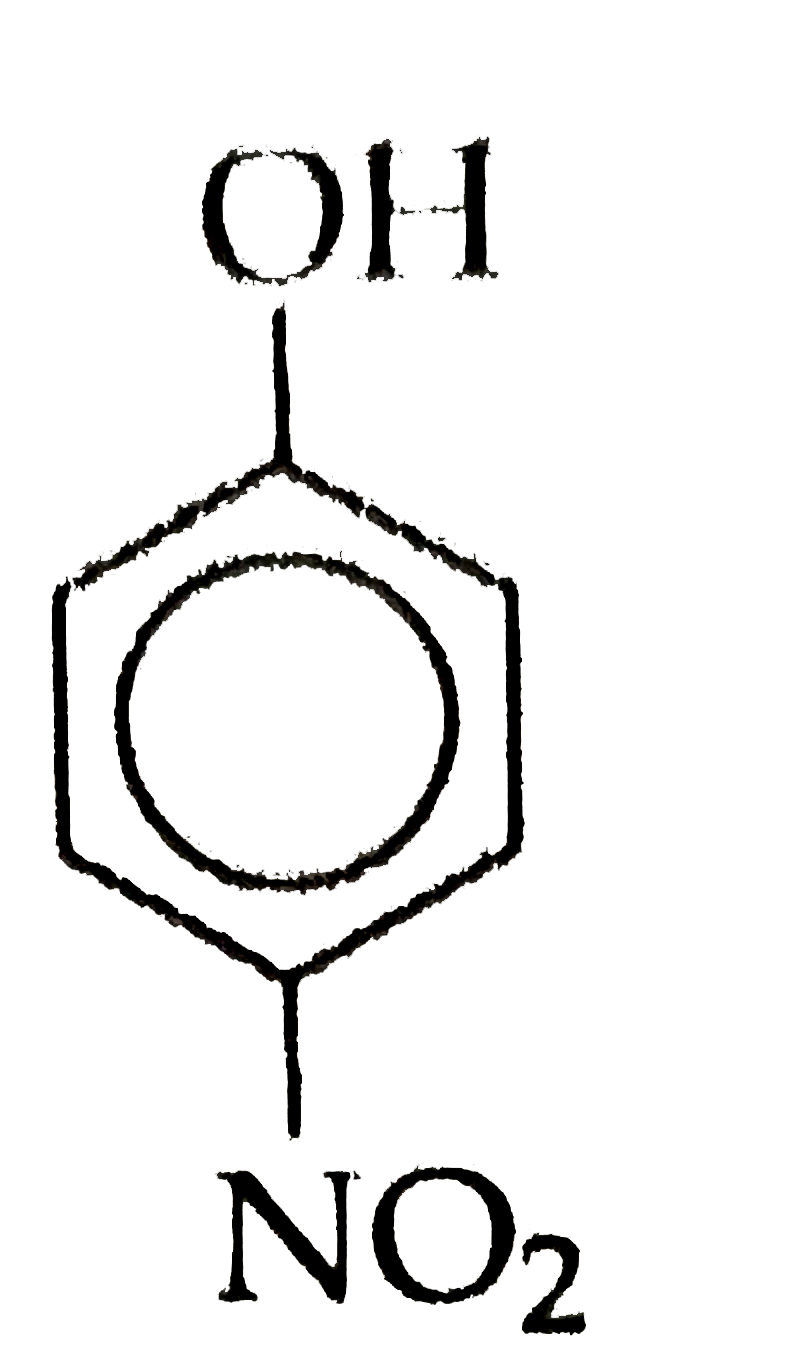
A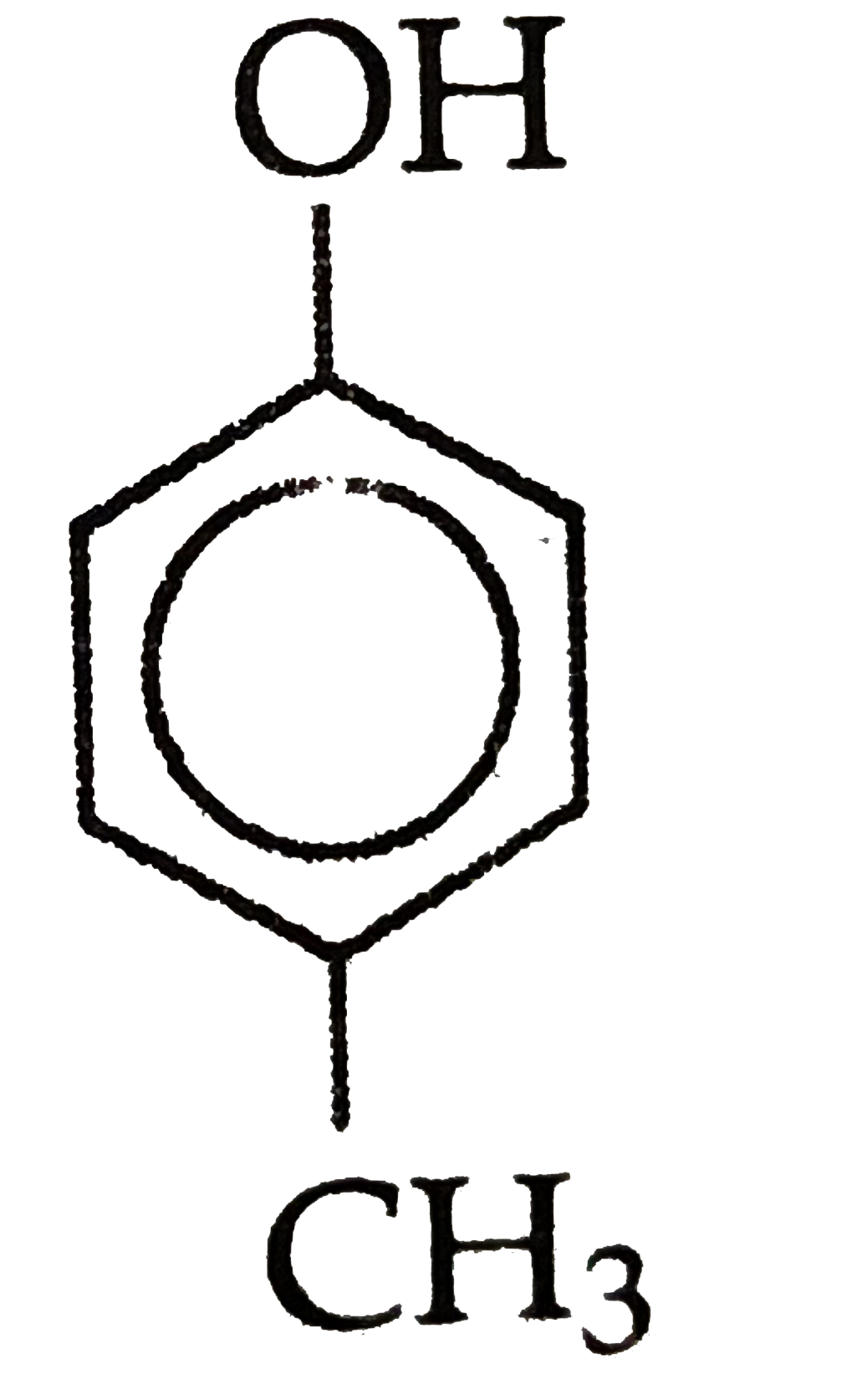 B
B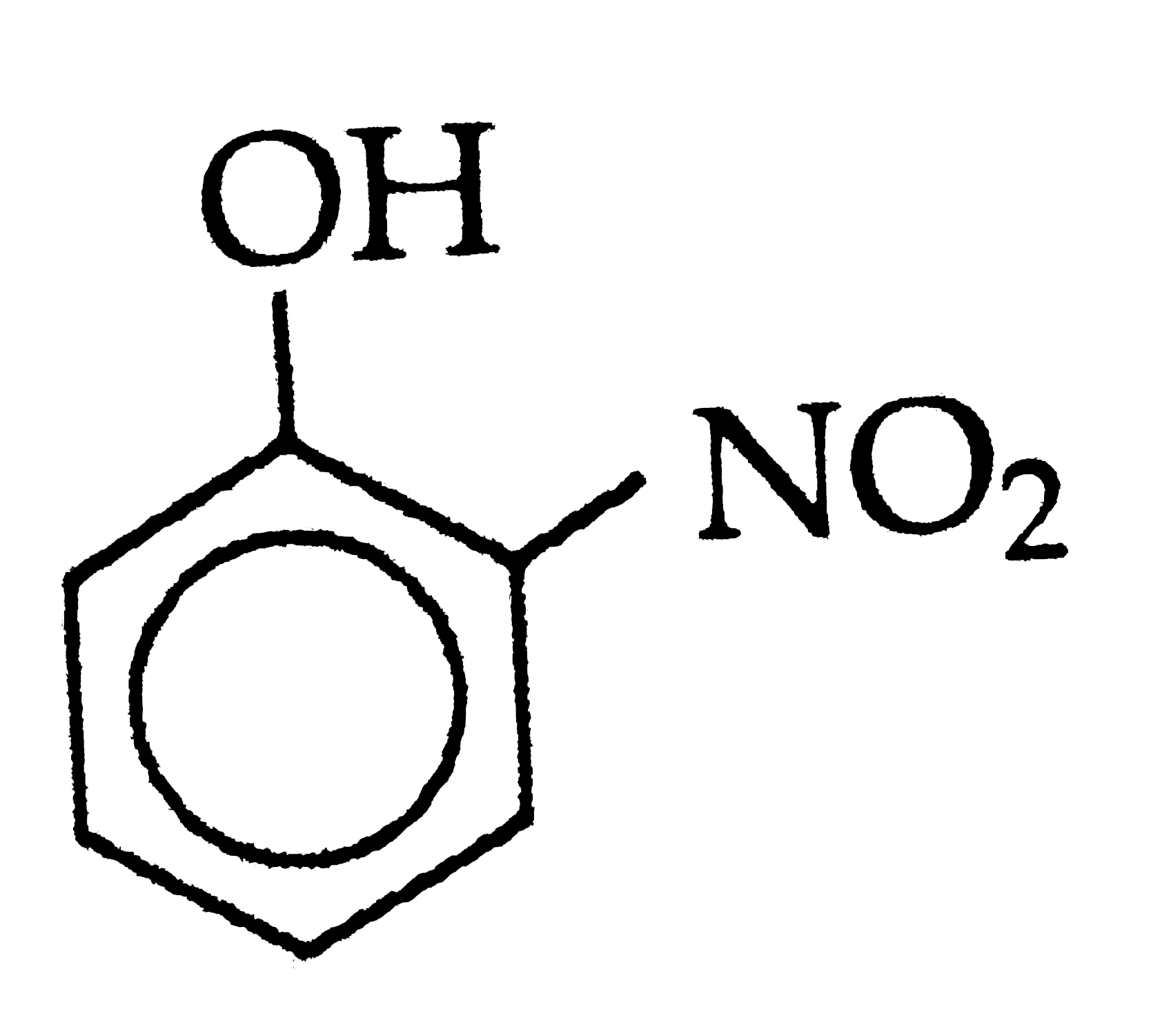 C
C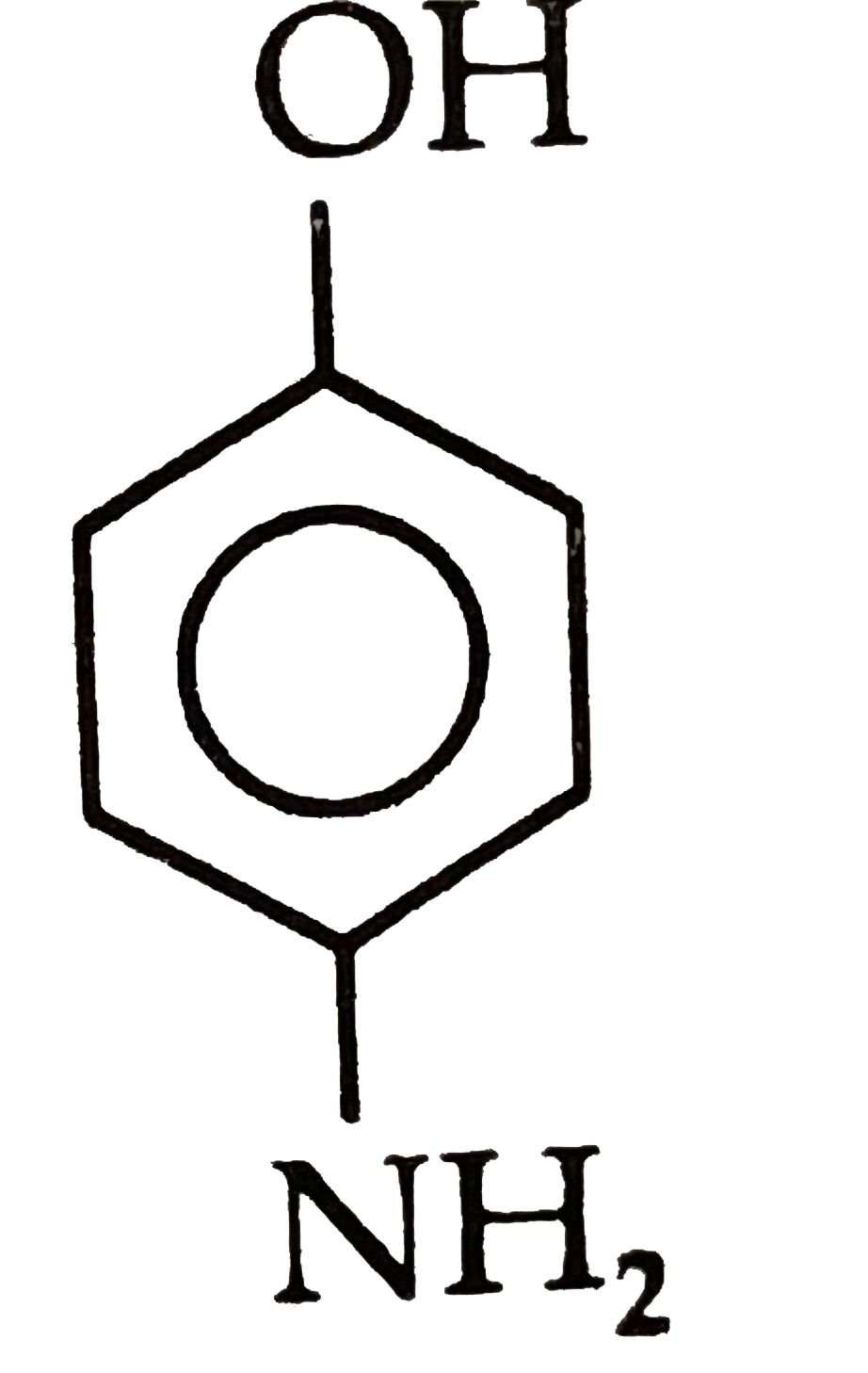 D
D