Which one of the following is most soluble ?
A
CuS(Ksp=8×10−37)
B
MnS(Ksp=7×10−16)
C
Bi2S3(Ksp=1×10−70)
D
Ag2S(Ksp=6×10−51)
Video Solution
Text Solution
The correct Answer is:B
Solubility is directly proportional to the Ksp.
|
Topper's Solved these Questions
- IONIC EQUILIBRIUMBook:ERRORLESS Chapter:IONIC EQUILIBRIUMExercise:Ordinary Thinking (Hydrogen ion concentration- pH scale and buffer solution )
Explore 225 Videos
- IONIC EQUILIBRIUMBook:ERRORLESS Chapter:IONIC EQUILIBRIUMExercise:Critical thinking (Objective question )
Explore 78 Videos
- IONIC EQUILIBRIUMBook:ERRORLESS Chapter:IONIC EQUILIBRIUMExercise:Ordinary Thinking (Acids and Bases)
Explore 154 Videos
- HYDROCARBONBook:ERRORLESS Chapter:HYDROCARBONExercise:JEE SECTION (Matrix Match type questions)
Explore 6 Videos
- NITROGEN CONTAINING COMPOUNDS Book:ERRORLESS Chapter:NITROGEN CONTAINING COMPOUNDS Exercise:JEE (ADVANCED) 2018 MORE THAN ONE CHOICE CORRECT ANSWER
Explore 1 Video
Similar Questions
Knowledge Check
Whhich one of the following is most soluble ?
ABi2S3(Ksp=1×10−70)BAg2S(Ksp=6×10−51)CCuS(Ksp=8×10−37)DMnS(Ksp=7×10−16)Which one of the following is most stable ?
ACH3−CH=CH−CH=CH−CH3BCH2=CH−CH=CH2CCH2=CH−CH2−CH=CH2DCH3−CH=CH−CH3Which one of the following is most stable ?
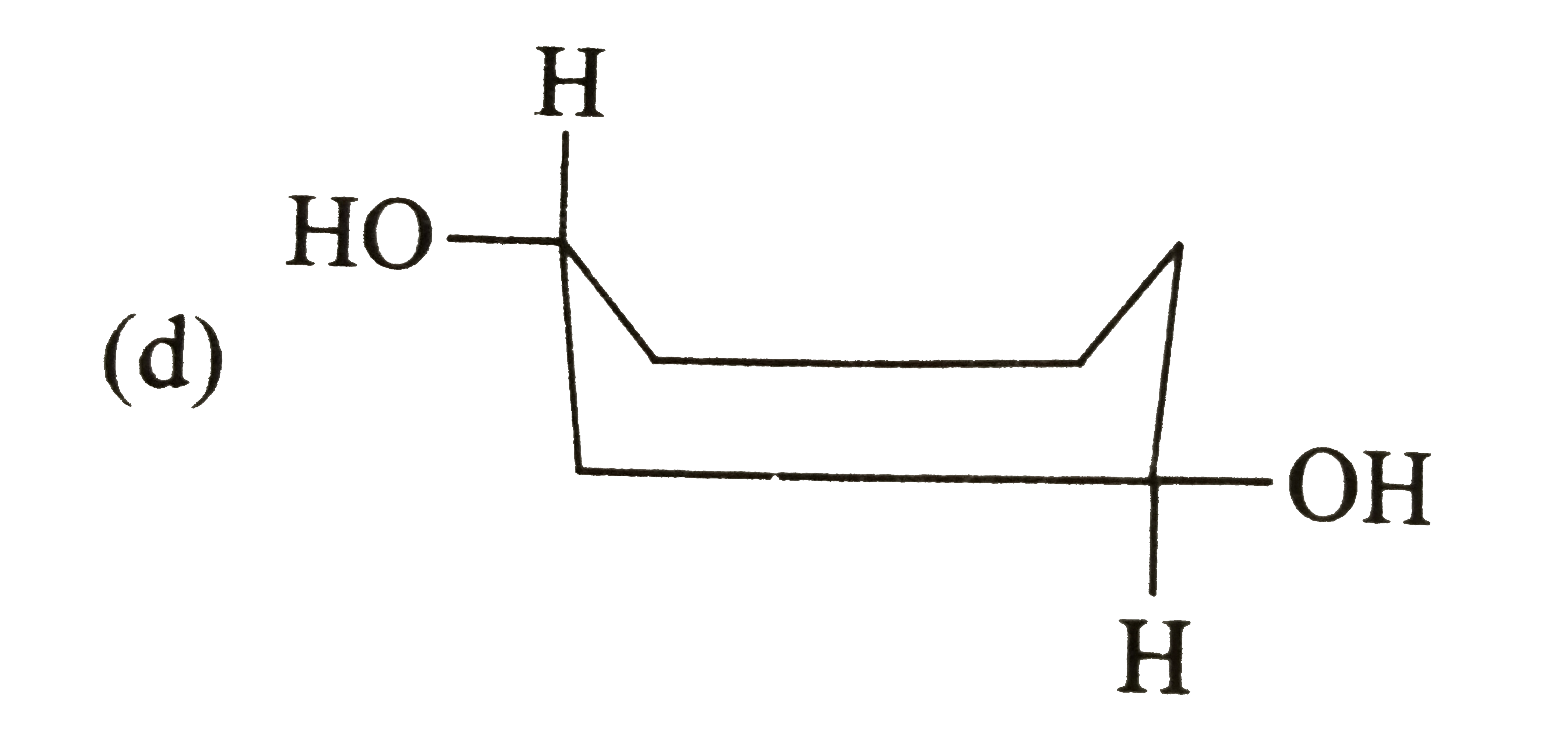
A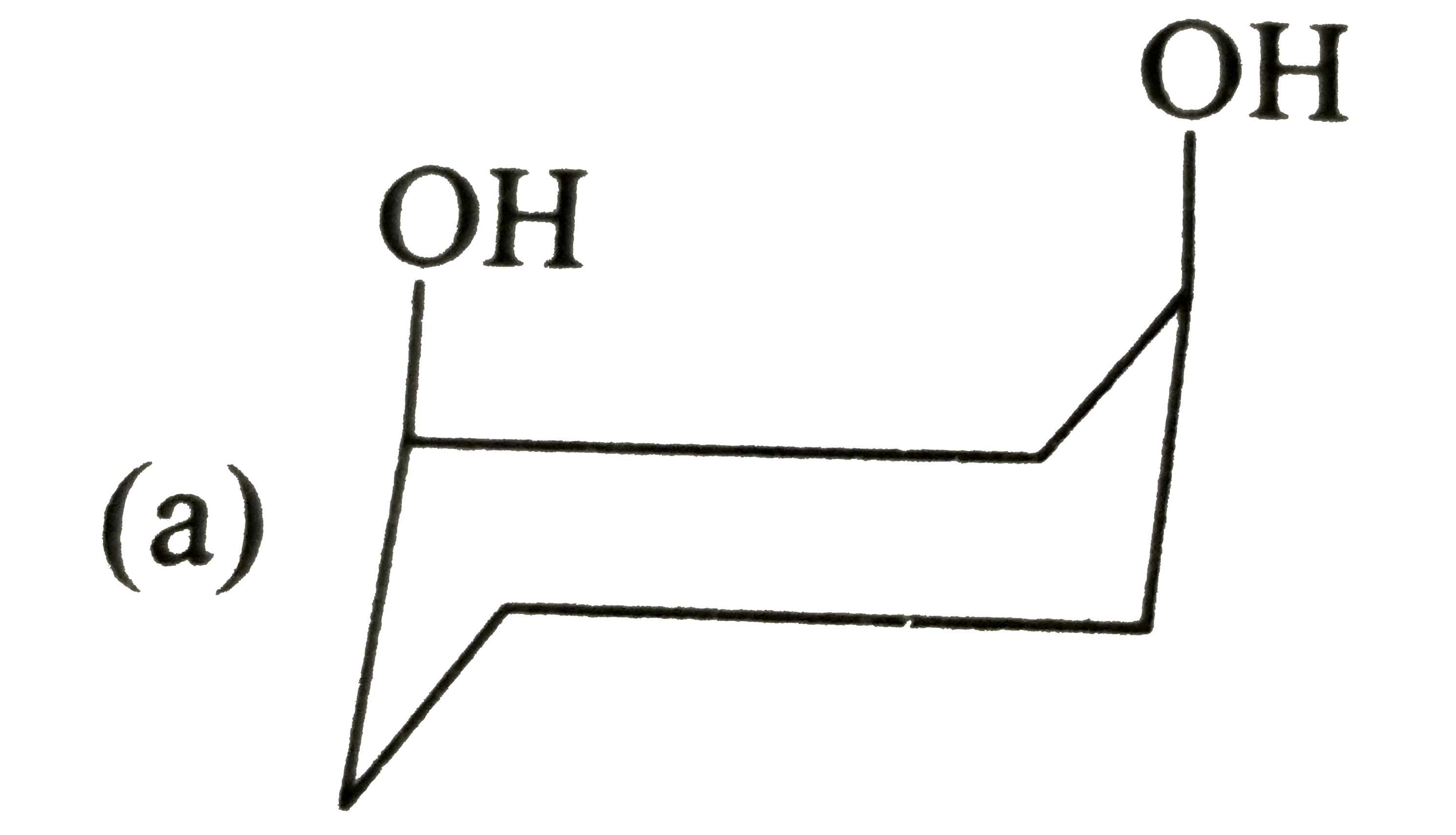 B
B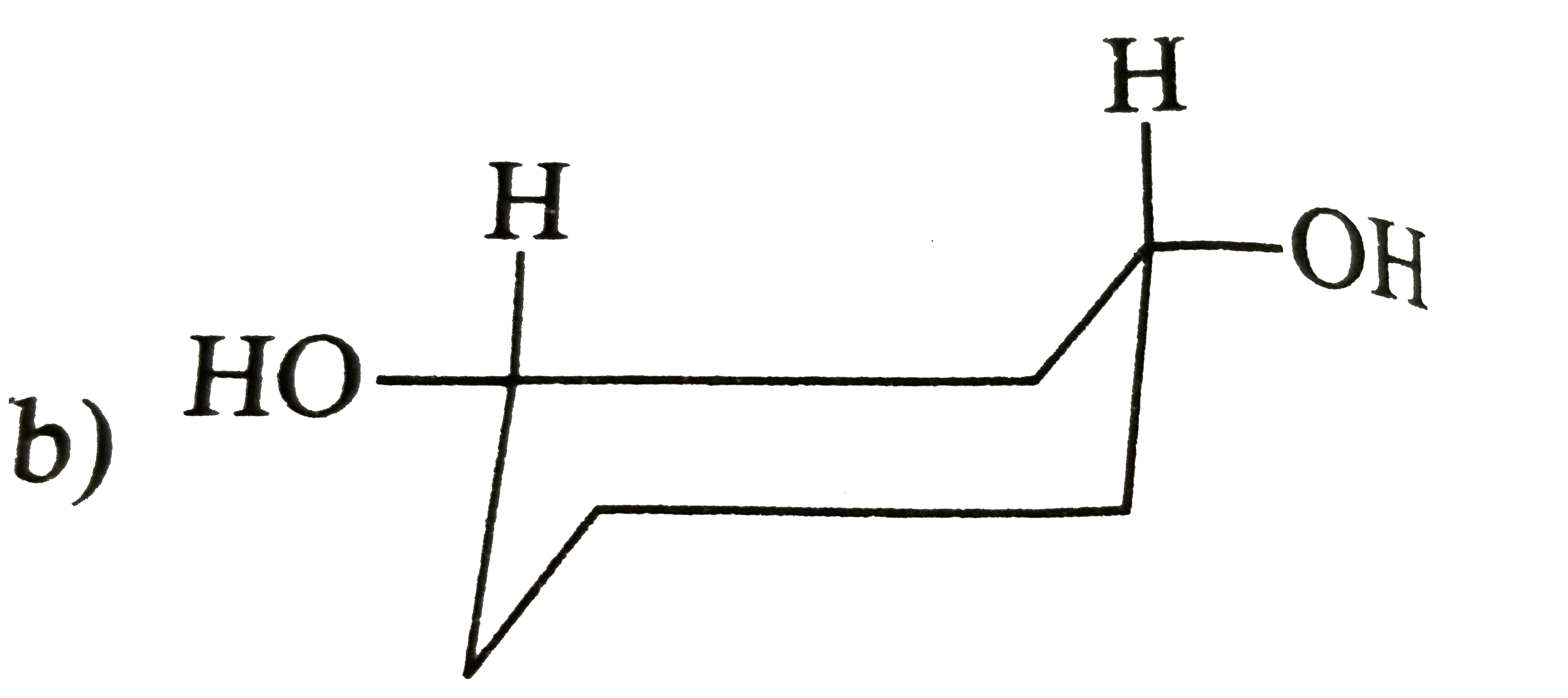 C
C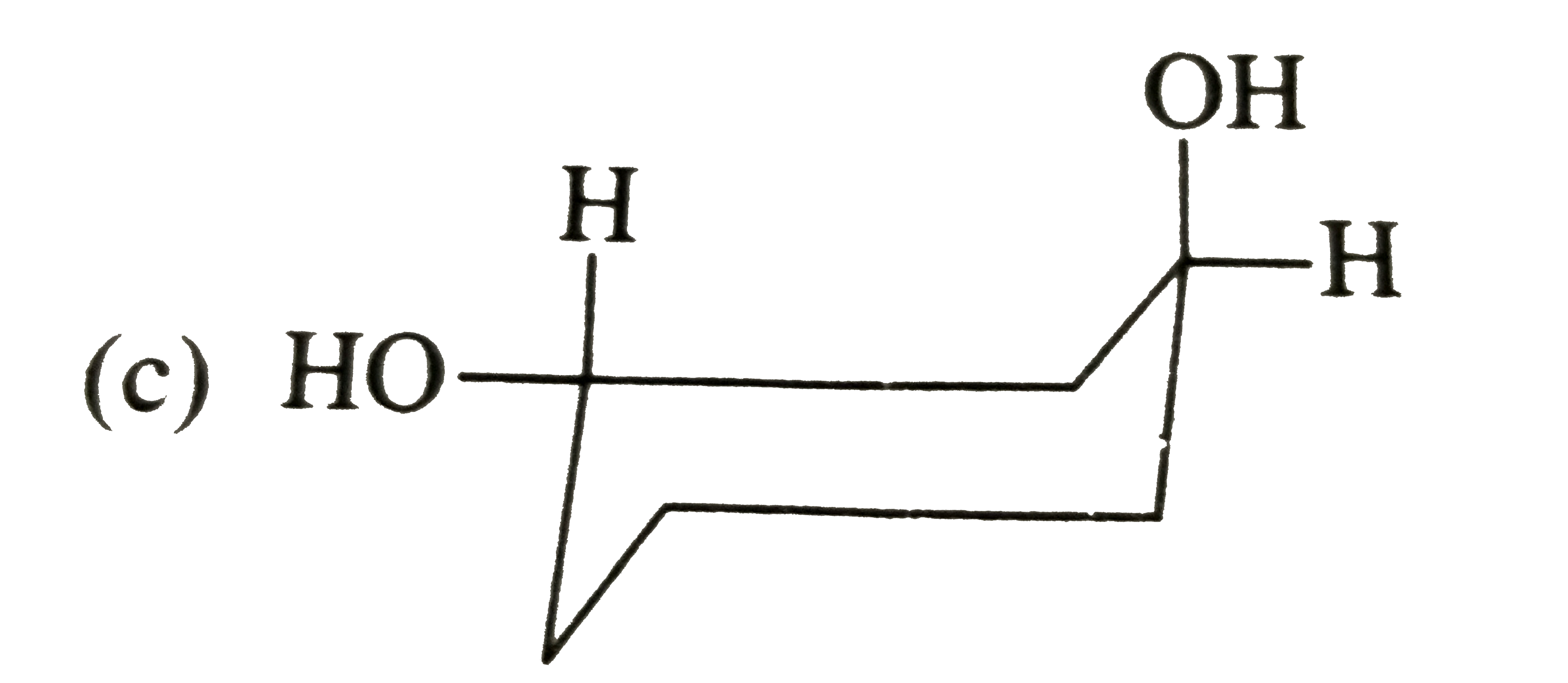 D
D