What is minimum concentration of SO2−4 required to precipitate BaSO4 in solution containing 1×10−4 mole of Ba2+ ? (Ksp of BaSO4=4×10−10)
4×10−10M
2×10−7M
4×10−6M
2×10−3M
The correct Answer is:C
BaSO4⇔Ba2++SO2−4
Ksp=S2⇒S=√Ksp,Ksp=[Ba2+]×[SO2−4]
4×10−10=[1×10−4]×[SO2−4]
[SO2−4]=4×10−101×104=4×10−6.
Topper's Solved these Questions
- IONIC EQUILIBRIUMBOOK - ERRORLESS CHAPTER - IONIC EQUILIBRIUMEXERCISE - Ordinary Thinking (Hydrogen ion concentration- pH scale and buffer solution )
225 Videos
- IONIC EQUILIBRIUMBOOK - ERRORLESS CHAPTER - IONIC EQUILIBRIUMEXERCISE - Critical thinking (Objective question )
78 Videos
- IONIC EQUILIBRIUMBOOK - ERRORLESS CHAPTER - IONIC EQUILIBRIUMEXERCISE - Ordinary Thinking (Acids and Bases)
154 Videos
- HYDROCARBONBOOK - ERRORLESS CHAPTER - HYDROCARBONEXERCISE - JEE SECTION (Matrix Match type questions)
6 Videos
- NITROGEN CONTAINING COMPOUNDS BOOK - ERRORLESS CHAPTER - NITROGEN CONTAINING COMPOUNDS EXERCISE - JEE (ADVANCED) 2018 MORE THAN ONE CHOICE CORRECT ANSWER
1 Video
Similar Questions
A buffer solution has equal volume of 0.20 M NH4OH and 0.02 MNH4Cl: The pKb of the base is 5. The pH is
View SolutionFor a weak acid HA with dissociation constant 10−9, POH of its 0.1 M solutions is
View SolutionThe degree of dissociation in a weak electrolyte increase
View SolutionEach of the compouns given in Column I with the reactions that they can undergo , given in column II .
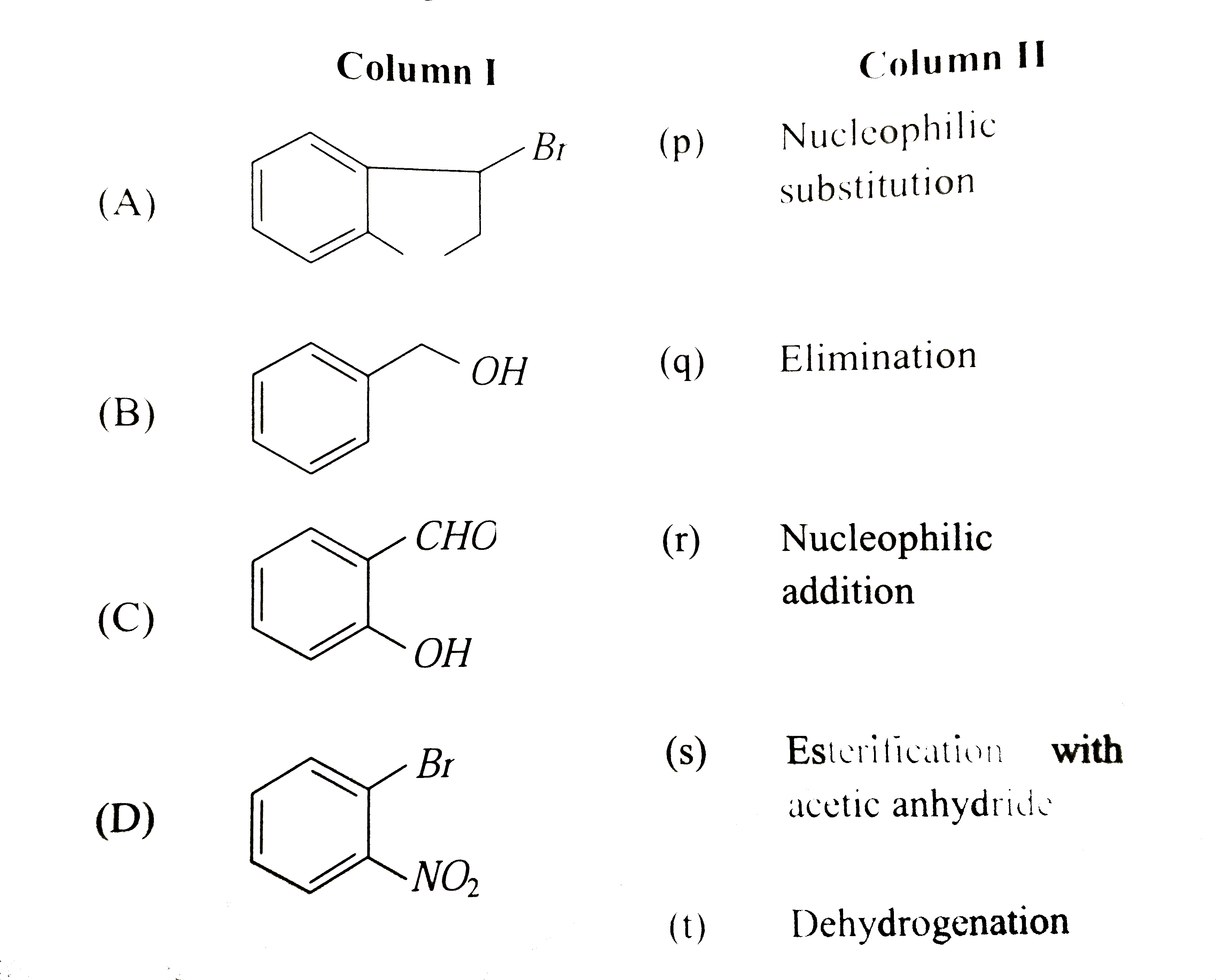
View SolutionAniline reacts with mixed acid (conc. HNO3 and conc. H2SO4) at 288K to give P(51%),Q(47%) and R (2%). The major product(s) of the following reaction sequence is (are)

View SolutionWhat is minimum concentration of SO_(4)^(2-) required to precipitate BaSO_(4) in solution containing 1 xx 10^(-4) mole of Ba^(2+) ? ( K_(sp) of BaSO_(4) = 4 xx 10^(-10) )
View SolutionOn adding 0.1 M solution each of Ag^(+),Ba^(2+), Ca^(2+) ions in a Na_(2)SO_(4) solution, species first precipitated is (K_(sp)BaSO_(4)=10^(-11),K_(sp)CaSO_(4)=10^(-6),K_(sp) Ag_(2)SO_(4)=10^(-5))
View SolutionThe solubility product of BaSO_(4) at 25^(@)C is 1.0 xx 10^(-9) . What would be the concentration of H_(2)SO_(4) necessary to precipitate BaSO_(4) from a solution of 0.01 M Ba^(2) ions
View SolutionWhat is the minimum concentration of Ba+2 ions required in order to initiate the precipitation of BaSO4 from a solution containing 0.002 mole L−1 of So−24 ions?
(Given Ksp for BaSO4=1.4×10−10)
View SolutionOn adding 0.1 M solution each of Ag^(+), Ba^(2+), Ca^(2+) ions in a Na_(2)SO_(4) solution, species first precipitated is (K_(sp)BaSO_(4)=10^(-11), K_(sp) CaSO_(4)=10^(-6), K_(sp)Ag_(2)SO_(4)=10^(-5))
View Solutionएक NaSO4 विलयन में [Ag+],[Ba2+],[Ca2+] प्रत्येक का 0.1 M विलयन मिलाने पर सबसे पहले अवक्षेपित होने वाली प्रजाति हैं:
[KSPBaSO4=10−11,KSPCaSO4=10−6,KSPAg2SO4=10−5]
View SolutionAn aqueous solution contains an unknown concentration of Ba2+ . When 50 mL of 1 M solution of Na2SO4 is added , BaSO4 just begins to precipitate . The final volume is 500 mL . The solubility product of BaSO4 is 1×10−10 . what is the original concentration of Ba2+ ?
View SolutionThe solubility product of BaSO4 at 25∘C is 1.0×10−9. What would be the concentration of H2SO4 necessary to precipitate BaSO4 from a solution of 0.01 M Ba+2 ions
View SolutionAn aqueous solution contains an unknown concentration of Ba2+. When 50 ml of a 1M solution of Na2SO4 is added. BaSO4just begins to precipitate. The final volume is 500ml. The solubility product ofBaSO4 is 1×10−10 Find the original concentration.
View SolutionKsp(BaSO4) is 1.1×1010. In which case is BaSO4 precipitated ?
View Solution

