Given a 0.1 M solution of each of the following. Which solution has the lowest pH
NaHSO4
NH4Cl
HCl
NH3
The correct Answer is:C
To determine which 0.1 M solution has the lowest pH, we need to analyze the nature of the solutions provided. The solutions typically consist of strong acids, weak acids, neutral salts, or strong bases.
1. Identify the Solutions:
Let's assume we have the following solutions for analysis:
- HCl (strong acid)
- CH₃COOH (weak acid)
- NaCl (neutral salt)
- NaOH (strong base)
2. Calculate pH for Each Solution:
- For HCl:
- HCl is a strong acid and completely dissociates in water.
- The concentration of H⁺ ions = 0.1 M.
- pH = -log(H⁺) = -log(0.1) = 1.
- For CH₃COOH:
- Acetic acid is a weak acid and does not completely dissociate.
- The dissociation constant (Ka) for acetic acid is approximately 1.8 x 10⁻⁵.
- Using the formula for weak acids:
(H⁺)=√Ka×C=√1.8×10−5×0.1≈0.00134 M
- pH = -log(0.00134) ≈ 2.87.
- For NaCl:
- Sodium chloride is a neutral salt and does not affect the pH significantly.
- pH ≈ 7.
- For NaOH:
- NaOH is a strong base and completely dissociates in water.
- The concentration of OH⁻ ions = 0.1 M.
- To find pH, first calculate pOH:
pOH=−log(OH⁻)=−log(0.1)=1.
- Then, use the relation pH + pOH = 14:
pH=14−pOH=14−1=13.
3. Compare the pH Values:
- HCl: pH = 1
- CH₃COOH: pH ≈ 2.87
- NaCl: pH ≈ 7
- NaOH: pH = 13
4. Conclusion:
The solution with the lowest pH is the 0.1 M HCl solution, which has a pH of 1.
Let's assume we have the following solutions for analysis:
- HCl (strong acid)
- CH₃COOH (weak acid)
- NaCl (neutral salt)
- NaOH (strong base)
- For HCl:
- HCl is a strong acid and completely dissociates in water.
- The concentration of H⁺ ions = 0.1 M.
- pH = -log(H⁺) = -log(0.1) = 1.
- For CH₃COOH:
- Acetic acid is a weak acid and does not completely dissociate.
- The dissociation constant (Ka) for acetic acid is approximately 1.8 x 10⁻⁵.
- Using the formula for weak acids:
(H⁺)=√Ka×C=√1.8×10−5×0.1≈0.00134 M
- pH = -log(0.00134) ≈ 2.87.
- For NaCl:
- Sodium chloride is a neutral salt and does not affect the pH significantly.
- pH ≈ 7.
- For NaOH:
- NaOH is a strong base and completely dissociates in water.
- The concentration of OH⁻ ions = 0.1 M.
- To find pH, first calculate pOH:
pOH=−log(OH⁻)=−log(0.1)=1.
- Then, use the relation pH + pOH = 14:
pH=14−pOH=14−1=13.
- HCl: pH = 1
- CH₃COOH: pH ≈ 2.87
- NaCl: pH ≈ 7
- NaOH: pH = 13
The solution with the lowest pH is the 0.1 M HCl solution, which has a pH of 1.
Similar Questions
Knowledge Check
Which solution has the highest pH ?
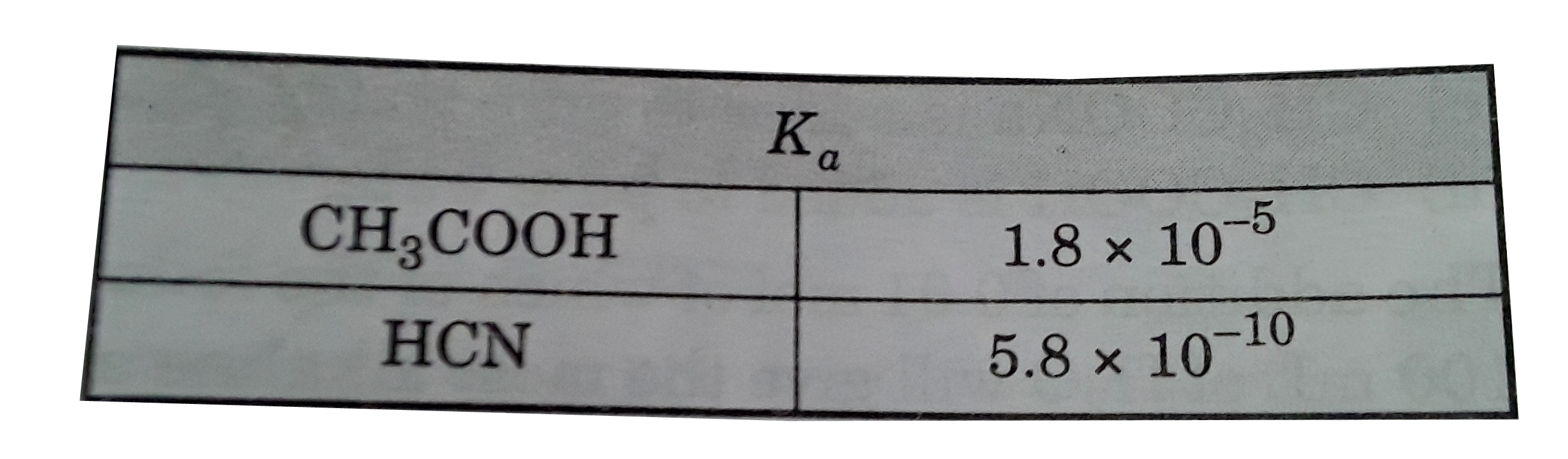 A0.10 M CH3COOHB0.10MHCNC0.10MCH3COOKD0.10MNaBr
A0.10 M CH3COOHB0.10MHCNC0.10MCH3COOKD0.10MNaBrWhich of the following solutions has the lowest pH value ?
A0 . 1 Molar NaCl solutionB0 .01 Molar NaHCO3 solutionC0 . 00 1 Molar Na2CO3 solutionD0.01 Molar NaOH solutionThe aqueous solution of which of the following salt will have the lowest pH ?
ANaClOBNaClO2CNaClO3DNaClO4
Which of the following solutions has pH=11 ?
View SolutionConsider 1M solutions of the following salts. State which solution will have the lowest freezing point.
View Solution0.1M solution of the following has the highest pH
View SolutionWhich of the following solutions has pH=12?
View SolutionWhich of the following 0.1M aqueous solution will have the lowest f.p. ?
View Solution
