0.023 g of sodium metal is reacted with 100cm3 of water. The pH of the resulting solution is
10
11
9
12
The correct Answer is:D
M=0.023×100023×100=0.01M
Na++oH−NaOH
[OH−]=0.01M=10−2M,pOH=−log(10−2)=2
pH+pOH=14
∴pH=14−2=12
Topper's Solved these Questions
- IONIC EQUILIBRIUMBook:ERRORLESS Chapter:IONIC EQUILIBRIUMExercise:Critical thinking (Objective question )
Explore 78 Videos
- IONIC EQUILIBRIUMBook:ERRORLESS Chapter:IONIC EQUILIBRIUMExercise:JS Jee Section (Only one choice correct answer)
Explore 48 Videos
- IONIC EQUILIBRIUMBook:ERRORLESS Chapter:IONIC EQUILIBRIUMExercise:Ordinary Thinking (Common ion effect, Isohydric solutions, Solubility product, Ionic product of water and Salt hydrolysis )
Explore 137 Videos
- HYDROCARBONBook:ERRORLESS Chapter:HYDROCARBONExercise:JEE SECTION (Matrix Match type questions)
Explore 6 Videos
- NITROGEN CONTAINING COMPOUNDS Book:ERRORLESS Chapter:NITROGEN CONTAINING COMPOUNDS Exercise:JEE (ADVANCED) 2018 MORE THAN ONE CHOICE CORRECT ANSWER
Explore 1 Video
Similar Questions
For a weak acid HA with dissociation constant 10−9, POH of its 0.1 M solutions is
View SolutionWhich of the following is the weakest base?
View SolutionThe hydrogen ion concentration in weak acid of dissociation constant Ka and concentration c is nearly equal to
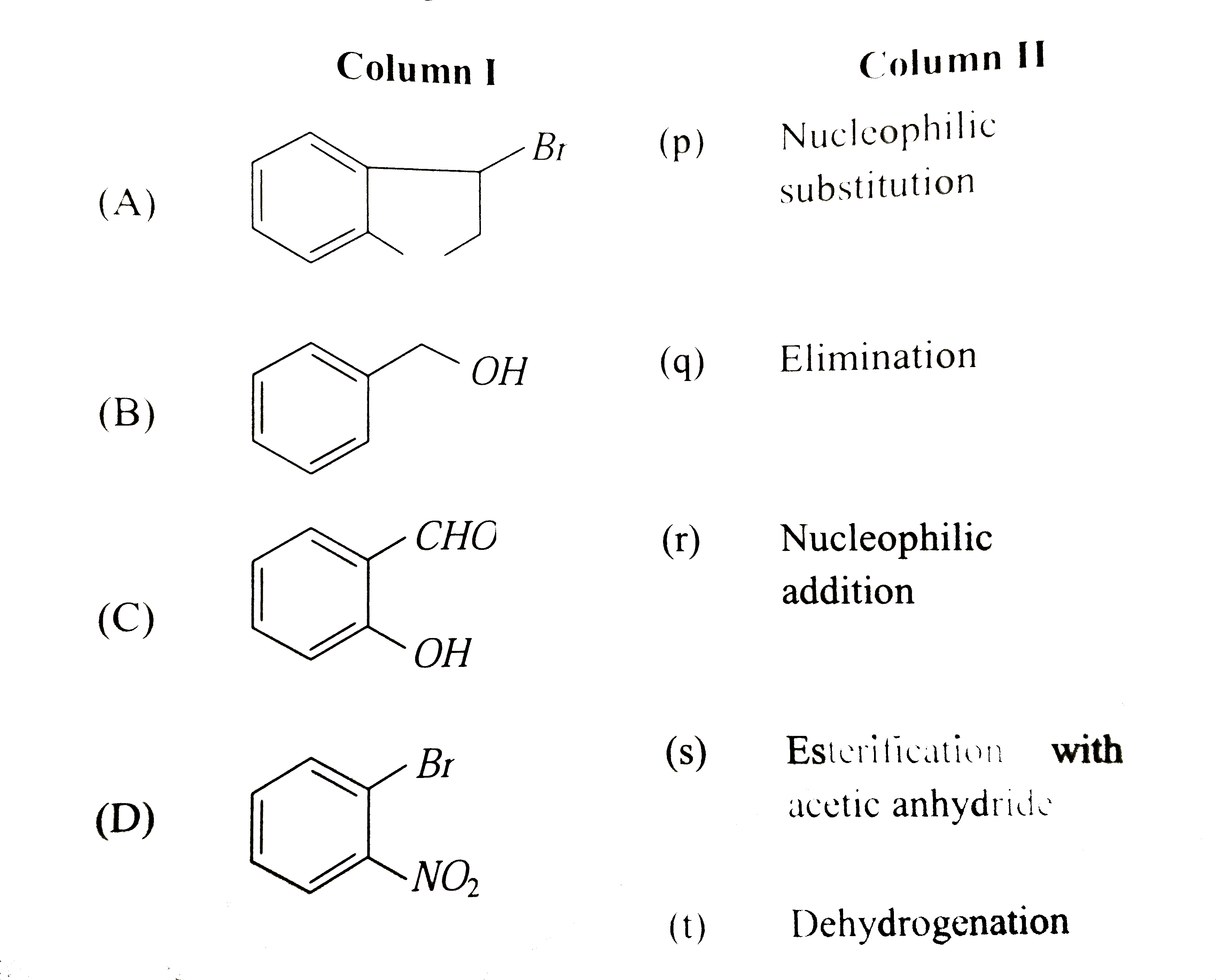
View SolutionEach of the compouns given in Column I with the reactions that they can undergo , given in column II .
View SolutionAniline reacts with mixed acid (conc. HNO3 and conc. H2SO4) at 288K to give P(51%),Q(47%) and R (2%). The major product(s) of the following reaction sequence is (are)

View Solution0.365g of HCl gas was passed through 100 cm3 of 0.2 M NaOH solution. The pH of the resulting solution would be
View Solution100 cc of HCl of pH value 1 is mixed with 100 cc of distilled water. The pH value of the resultant solution is
View Solution0.023 g of sodium metal is reacted with 100 cm^(3) of water. The pH of the resulting solution is
View Solutionसोडियम धातु पानी से अभिक्रिया करती है ।
View Solution1cm3 of 0.01 N HCl solution is added to one litre of sodium chloride solution . Calculate the pH of the resulting solution.
View Solution0.365g of HCl gas was passed through 100 cm3 of 0.2 M NaOH solution. The pH of the resulting solution would be
View Solution0.023 g of sodium metal is reacted with 100cm3 of water. The pH of the resulting solution is
View Solution0.023 g सोडियम धातु की 100cm3 जल में क्रिया कराई गई ओट बने विलयन की pH हैं:
View Solution0.023g of sodium metal is reacted with 100cm3 of water. The pH of the resulting solution is :
View Solution0.023g of sodium metal is reacted with 100cm3 of water. The pH of the resulting solution is :
View Solution
