Which solutions has the highest pH value
1M KOH
1MH2SO4
Chlorine water
Water containing carbon dioxide
The correct Answer is:A
To determine which solution has the highest pH value, we need to understand the pH scale and the nature of the solutions provided. Here’s a step-by-step solution:
1. Understanding pH Scale:
- The pH scale ranges from 0 to 14.
- A pH of 7 is considered neutral (pure water).
- pH values less than 7 indicate acidic solutions.
- pH values greater than 7 indicate basic (alkaline) solutions.
2. Identifying Acidic and Basic Solutions:
- Acids have a pH less than 7. Examples include sulfuric acid (H₂SO₄) and hydrochloric acid (HCl).
- Bases have a pH greater than 7. For example, sodium hydroxide (NaOH) is a common base.
3. Analyzing Given Solutions:
- If the options include acids and at least one base, the base will have the highest pH.
- For instance:
- H₂SO₄ (sulfuric acid) has a pH < 7.
- HCl (from chlorine water) has a pH < 7.
- Water containing CO₂ forms carbonic acid (H₂CO₃), which also has a pH < 7.
- If there is a base (e.g., NaOH), it will have a pH > 7.
4. Conclusion:
- Since bases have pH values greater than 7, the solution that is a base will have the highest pH value compared to the acidic solutions.
Final Answer:
The solution with the highest pH value is the one that is a base.
- The pH scale ranges from 0 to 14.
- A pH of 7 is considered neutral (pure water).
- pH values less than 7 indicate acidic solutions.
- pH values greater than 7 indicate basic (alkaline) solutions.
- Acids have a pH less than 7. Examples include sulfuric acid (H₂SO₄) and hydrochloric acid (HCl).
- Bases have a pH greater than 7. For example, sodium hydroxide (NaOH) is a common base.
- If the options include acids and at least one base, the base will have the highest pH.
- For instance:
- H₂SO₄ (sulfuric acid) has a pH < 7.
- HCl (from chlorine water) has a pH < 7.
- Water containing CO₂ forms carbonic acid (H₂CO₃), which also has a pH < 7.
- If there is a base (e.g., NaOH), it will have a pH > 7.
- Since bases have pH values greater than 7, the solution that is a base will have the highest pH value compared to the acidic solutions.
The solution with the highest pH value is the one that is a base.
Similar Questions
Knowledge Check
Which solution has the highest pH ?
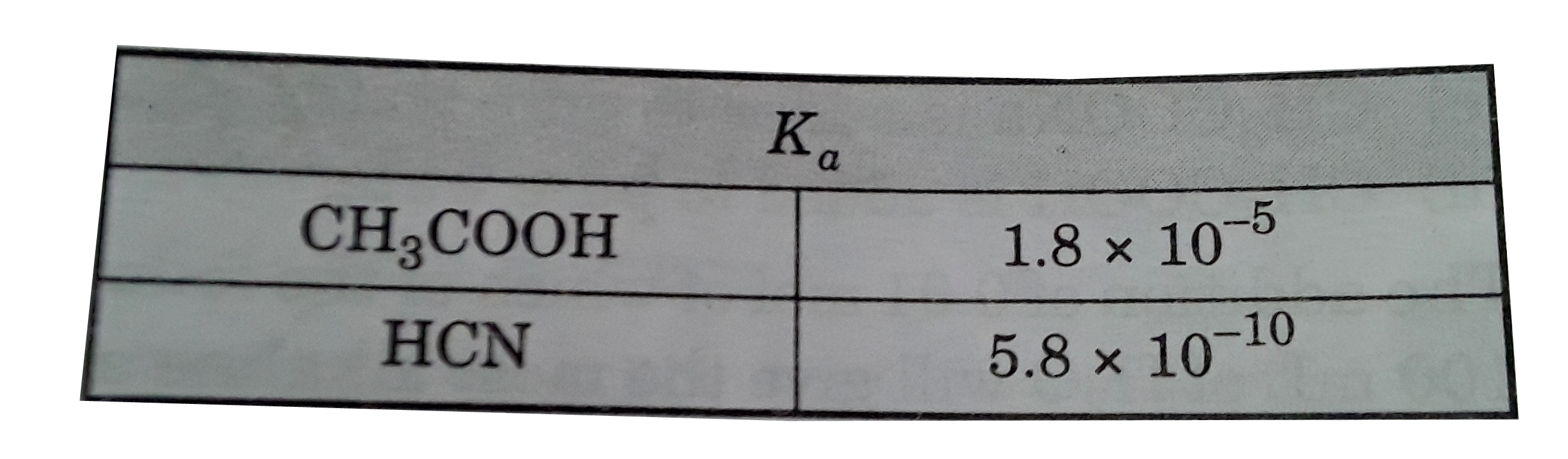 A0.10 M CH3COOHB0.10MHCNC0.10MCH3COOKD0.10MNaBr
A0.10 M CH3COOHB0.10MHCNC0.10MCH3COOKD0.10MNaBrWhich has the highest pH?
ACH3COOKBNa2CO3CNH4ClDNaNO3The pH of the solutions are given. Which of the solutions has the highest concetration of hydrogen ions?
A10.1B4.7C2.4D8
Which of the following solutions has the highest equivalent conductance?
View SolutionA compound whose solution has the highest pH is
View SolutionThe pH of the solutions are given. Which of the solutions has the hiehest concentration of hydrogen ions ?
View SolutionWhich compound has the highest value of pKa ?
View SolutionWhich has the highest pKb value?
View Solution

