The correct relation between equilibrium constant (K), standard free energy (ΔG∘) and temperature (T) is
ΔG∘=RTlnK
K=e−ΔG∘/2.303RT
ΔG∘=−RTlog10K
K=10−ΔG∘/2.303RT
The correct Answer is:D
To find the correct relation between the equilibrium constant (K), standard free energy change (ΔG°), and temperature (T), we can follow these steps:
1. Understand the Gibbs Free Energy Equation:
The Gibbs free energy change (ΔG) for a reaction can be expressed as:
ΔG=ΔG∘+RTlnQ
where:
- ΔG is the Gibbs free energy change at any point.
- ΔG° is the standard Gibbs free energy change.
- R is the universal gas constant (8.314 J/(mol·K)).
- T is the temperature in Kelvin.
- Q is the reaction quotient.
2. Apply the Condition at Equilibrium:
At equilibrium, the Gibbs free energy change (ΔG) is zero, and the reaction quotient (Q) becomes the equilibrium constant (K):
0=ΔG∘+RTlnK
3. Rearranging the Equation:
Rearranging the equation gives:
ΔG∘=−RTlnK
4. Convert Natural Logarithm to Base 10:
To express K in terms of base 10 logarithm, we can use the conversion between natural logarithm and logarithm base 10:
lnK=2.303logK
Substituting this into the equation gives:
ΔG∘=−RT(2.303logK)
5. Final Relation:
Rearranging this gives us the final relation:
logK=−ΔG∘2.303RT
This indicates that the logarithm of the equilibrium constant K is directly related to the standard free energy change ΔG° and inversely related to the temperature T.
Conclusion:
The correct relation between the equilibrium constant (K), standard free energy change (ΔG°), and temperature (T) is:
logK=−ΔG∘2.303RT
The Gibbs free energy change (ΔG) for a reaction can be expressed as:
ΔG=ΔG∘+RTlnQ
where:
- ΔG is the Gibbs free energy change at any point.
- ΔG° is the standard Gibbs free energy change.
- R is the universal gas constant (8.314 J/(mol·K)).
- T is the temperature in Kelvin.
- Q is the reaction quotient.
At equilibrium, the Gibbs free energy change (ΔG) is zero, and the reaction quotient (Q) becomes the equilibrium constant (K):
0=ΔG∘+RTlnK
Rearranging the equation gives:
ΔG∘=−RTlnK
To express K in terms of base 10 logarithm, we can use the conversion between natural logarithm and logarithm base 10:
lnK=2.303logK
Substituting this into the equation gives:
ΔG∘=−RT(2.303logK)
Rearranging this gives us the final relation:
logK=−ΔG∘2.303RT
This indicates that the logarithm of the equilibrium constant K is directly related to the standard free energy change ΔG° and inversely related to the temperature T.
The correct relation between the equilibrium constant (K), standard free energy change (ΔG°), and temperature (T) is:
logK=−ΔG∘2.303RT
Similar Questions
Knowledge Check
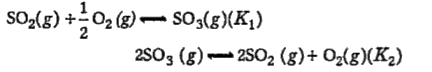
The correct relationship between equilibrium constantsK1 and K2 isAK1=1K2BK1=1√K2CK1=K2Dnone of theseThe relationship between equilibrium constants KpandKc for a gaseous reaction is:
AKc=Kp(RT)ΔnBKp=Kc(RT)ΔnCKp=(KcRT)ΔnDKp=Kc=(RT)ΔnThe free energy change in related to equilibrium constant as
AΔG=RT in kcB−ΔG=RTlogkcC−ΔG=2.303RtlogkcD−ΔG=(RTlogkc)/2.303.
The free energy change is related to equilibrium constant as
View SolutionThe equilibrium constant of a reaction is 0.008 at 298K. The standard free energy change of the reaction at the same temperture is
View SolutionWhich is the correct relationship between ΔG∘ and equilibrium constant Kp?
View SolutionIf equilibrium constant for a reaction is K, then standard free energy change is :
View SolutionThe relationship between the free energy change (ΔG) and entropy change (ΔS) at constant temperature (T) si
View Solution
