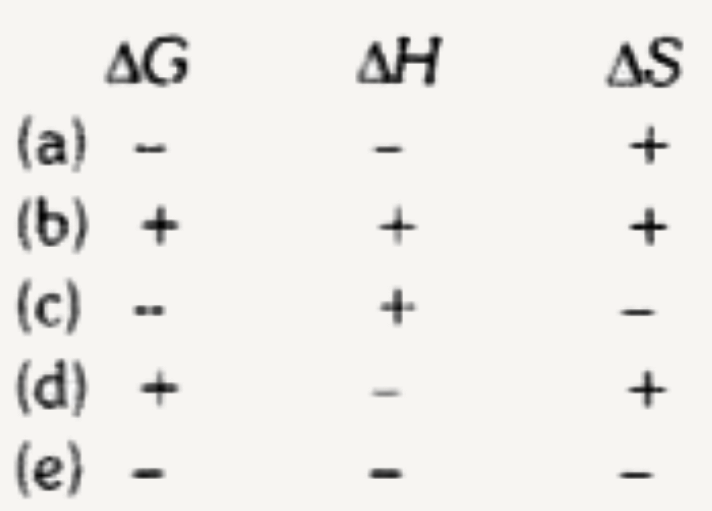
A chemical reaction is spontancous at 298K but non spontaneous at 350K . Which one of the following is true for the reaction ?
ΔGΔHΔS__+
ΔGΔHΔS+++
ΔGΔHΔS-+-
ΔGΔHΔS---
The correct Answer is:D
ΔG=ΔH−TΔS
For first condition, reaction will be spontaneous at all temperatures.
For second option as ΔH=+ve and ΔS is +ve thus DeltaG=underset(+ve)underbrace(DeltaH)-underset((-ve))underbrace(TDeltaS)
If temperature is high, TΔS will have higher negative value and reaction will be spontaneous
Thus not true for given reaction.
In case (c), ΔG=ve, ΔS=+ve
implies spontaneous at all temperatures
In case (e), ΔG=ΔH−ve−TΔS+ve
At high temperature, second term will have high positive value and reaction will be non-spontaneous, thus case (e) is true for given example.
Similar Questions
Which of the following statement(s) is /are correct ?
(i) ΔG is negative, then the reaction is spontaneous and proceeds in the forward direction.
(ii) ΔG is positive, then reaction is non-spontancous
(iii) ΔG is 0, then reaction is at equilibrium
View Solutionपरम ताप , 298 K में 10 K की वृद्धि होने पर रासायनिक अभिक्रिया का वेग दुगुना हो जाता है । इस अभिक्रिया के लिये Eaकी गणना कीजिये ।
View SolutionPredict which of the following reactions would proceed spontaneously at 298 K? [Hint : Refer to Electrochemical series]
View SolutionA reaction is spontaneous at low temperature but non- spontaneous at high temperature. Which of the following is true for the reaction?
View Solution................... Is a process in which electrical energy is used to cause a non-spontaneous chemical reaction.
View Solution298 (K) पर मेथेनॉल का ΔH∘f निम्न रासायनिक अभिक्रिया द्वारा दिया गया है
View Solutionएक रासायनिक अभिक्रिया 298K ताप पर स्वतः परंतु 350K ताप पर अस्वतः होती है। निम्न में से अभिक्रिया के लिए सत्य है
View SolutionReaction N_2O_4(g) harr 2NO_2 (g) For 298 K K_P The value of is 0.98. Mention whether the reaction is spontaneous or not.
View Solution

