The essential condition for the feasibility of a reaction is that __________ .
A
The reaction should be exothermic
B
The entropy of products must be larger than that of reactants
C
The reaction is to be accompanied with free energy decrease
D
The reaction has to possess high activation energy
Video Solution
Text Solution
The correct Answer is:C
If DeltaG=-ve, reaction is spontaneous.
|
Similar Questions
Knowledge Check
The essential condition for demodulation is
Avc<<RCB1vc<<RCC1vc>>RCDvc>>RCThe essential condition for demodulation is
Avc<<RCB1vc<<RCC1vc>>RCDvc>>RCThe feasible reaction is :
ACH3COOH+NaCl→BC6H5COOH+KBr→C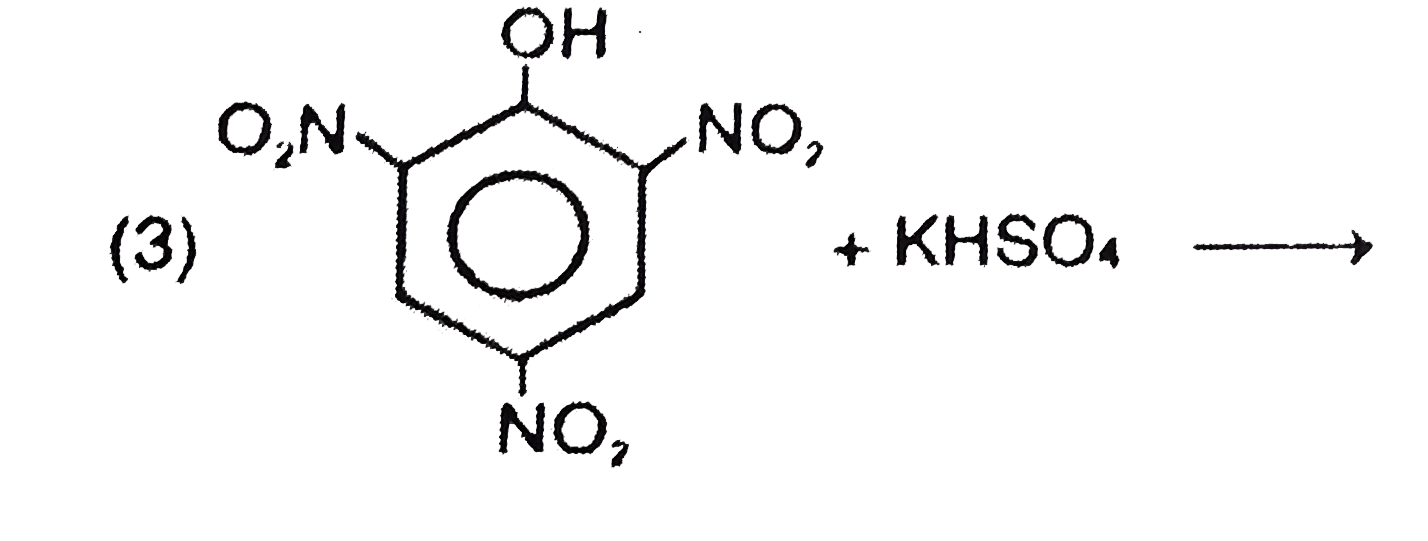 D
D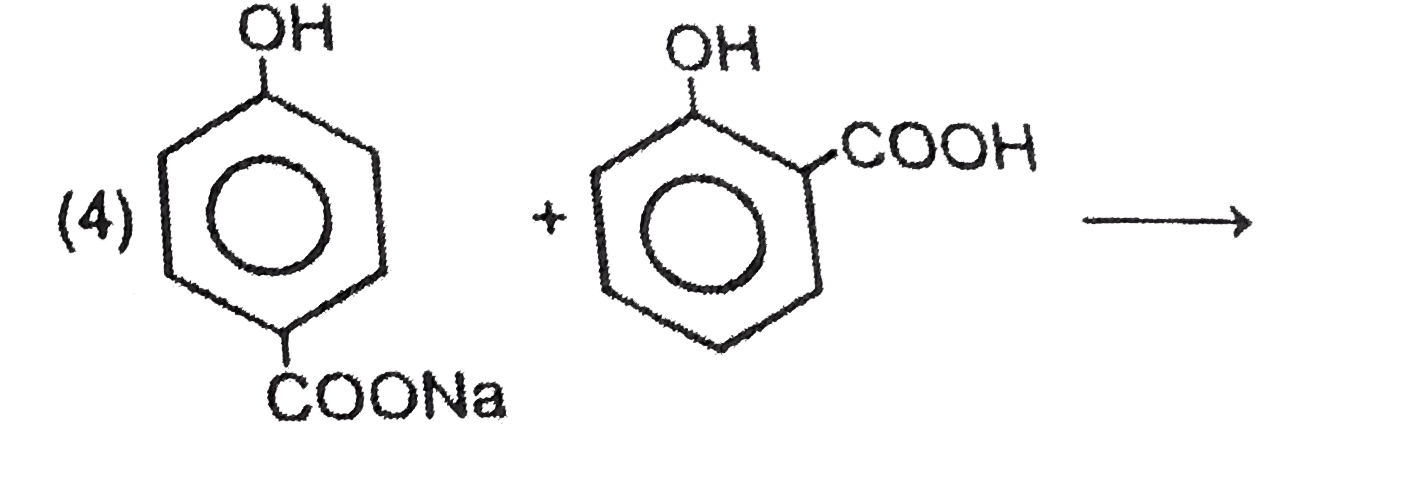
State the essential condition for the addition of vectors.
View SolutionWhat is the essential condition for the formation of beats?
View SolutionMention essential conditions for origin of life
View SolutionEssential Condition For Isothermal Process
View SolutionWhat is the thermodynamic criteria for the feasibility of a reaction ?
View Solution
