Using the data provided, calculate the multiple bond energy (kJmol−1) of a C≡C bond in C2H2. That energy is ( take the bond energy of a C−H bond as 350kJmol−1).
2C(s)+H2(g)→C2H2(g),Δ=225kJmol−1
2C(s)→2Cg),ΔH=1410kJmol−1
H2(g)→2H(g),ΔH=330kJmol−1
1165
837
865
815
The correct Answer is:D
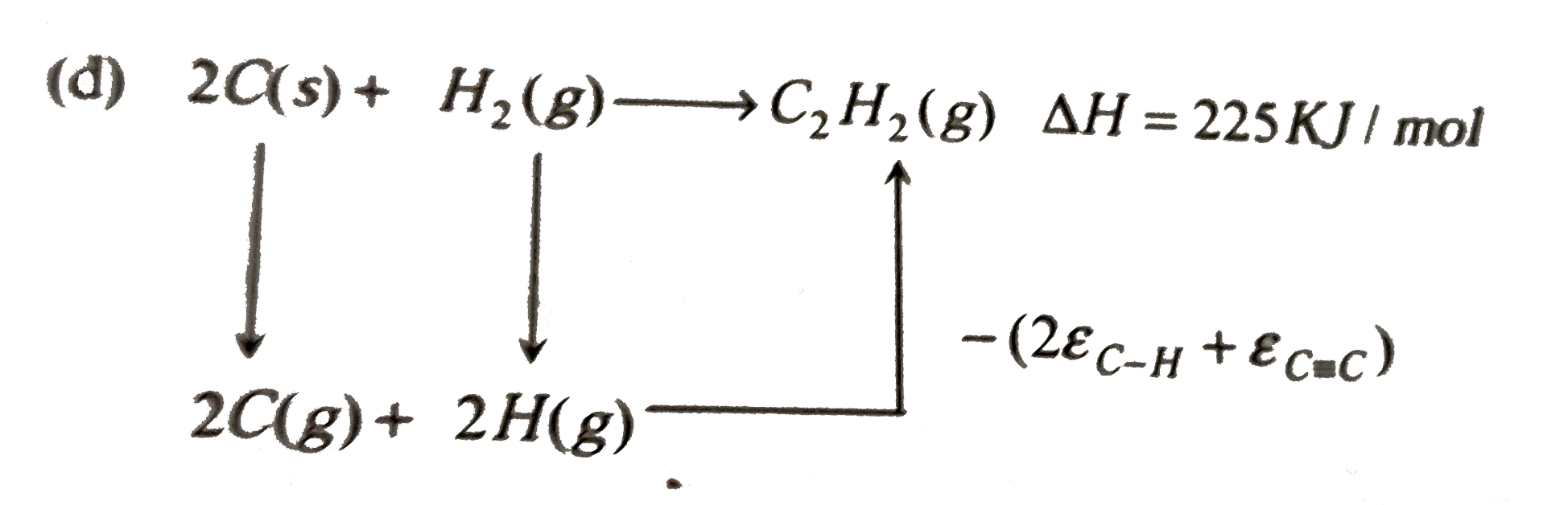
∴ΔH=+1410+330−(350×2)−εC≡C=+225
∴εC≡C=1740−1700−225=+815kJ/mol.
Similar Questions
From the following thermochemical equations, calculate the standard enthalpy of formation of HCl(g) .
(A) H2(g)→2H(g),ΔH0=+436.0kJmol−1 (B)Cl2(g)→2Cl(g),ΔH0=+242.7kJmol−1
(C ) HCl(g)→H(g)+Cl(g),ΔH0=+431.8kJmol−1
View SolutionUsing the data provided, calculate the multiple bond energy (kJmol−1) of a C≡C bond in C2H2.That energy is (take the bond enrgy of a C-H bond as 350 kJ mol−1):
2C(s)+H2(g)→C2H2(g),ΔH=225kJmol−1
2C(s)→2C(g),ΔH=1410kJmol−1
H2(g)→2H(g),ΔH=330kJmol−1
View Solution
Knowledge Check
Using the data provided, calculate the multiple bond energy (kJmol−1) of a C≡C bond in C2H2. That energy is ( take the bond energy of a X−H bond as 350kJmol−1).
2C(s)+H2(g)→C2H2(g),Δ=225kJmol−1
2C(g)→2Cg),ΔH=1410kJmol−1
H2(g)→2H(g),ΔH=330kJmol−1A1165B837C865D815Using the data provided, calculate the multiple bond energy (kJmol−1) of a C≡C bond in C2H2. That energy is ( take the bond energy of a C−H bond as 350kJmol−1).
2C(s)+H2(g)→C2H2(g),Δ=225kJmol−1
2C(s)→2Cg),ΔH=1410kJmol−1
H2(g)→2H(g),ΔH=330kJmol−1A1165B837C865D815Using the data provided, calculate the multiple bond energy (kJmol−1) of a C≡C bond in C2H2.That energy is (take the bond enrgy of a C-H bond as 350 kJ mol−1):
2C(s)+H2(g)→C2H2(g),ΔH=225kJmol−1
2C(s)→2C(g),ΔH=1410kJmol−1
H2(g)→2H(g),ΔH=330kJmol−1A1165B837C865D815
Using the data provided, calculate the multiple bond energy (kJ mol−1 ) of a C≡C bond in C2H2 . That energy is (take the bond energy of a C- H bond as 350 kJ mol−1)
2C(s)+H2(g)→C2H2(g)ΔH=225kJmol−1
2C(s)→2C(g) ΔH=1410kJmol−1
H2(g)→2H(g) ΔH=330kJmol−1
View SolutionUsing the data provided, calculate the multiple bond energy (kJmol−1) of a C≡C bond in C2H2. That energy is ( take the bond energy of a C−H bond as 350kJmol−1).
2C(s)+H2(g)→C2H2(g),Δ=225kJmol−1
2C(s)→2Cg),ΔH=1410kJmol−1
H2(g)→2H(g),ΔH=330kJmol−1
View SolutionUsing the data provided, calculate the mutliple bond energy (kJ mol−1) of a C≡C bond in C2H2. That energy is (take the bond energy of a C-H bond as 350 kJ mol−1)
2C(s)+H2(g)→HC≡CH(g),ΔH=225kJmol−1
2C(s)→2C(g),Δ=1410kJ mol−1
H2(g)→2H(g),ΔH=330kJ mol−1
View SolutionUsing the data provided, calculate the multiple bond energy (kJmol−1) of a C≡C bond in C2H2.That energy is (take the bond enrgy of a C-H bond as 350 kJ mol−1):
2C(s)+H2(g)→C2H2(g),ΔH=225kJmol−1
2C(s)→2C(g),ΔH=1410kJmol−1
H2(g)→2H(g),ΔH=330kJmol−1
View SolutionThe average O-H bond energy in H2O with the help of following data.
(1) H2O(l)→H2O(g),ΔH=+40.6kJmol−1
(2) 2H(g)→H2(g),ΔH=−435.kJmol−1
(3) O2(g)→2O(g),ΔH=+489.6kJmol−1
(4) 2H2(g)+O2(g)→2H2O(l),ΔH=−571.6kJmol−1
View Solution
