Which of the statement (a)- (d) about the reaction profile below is false

A
The product is more stable than the reactant
B
The second step is rate determining
C
The reaction is exothermic
D
The equilibrium constant is greater than 1 if the molar entropy change is negligible
Text Solution
The correct Answer is:b
The second step is fast step due to low Ea
|
Similar Questions
Knowledge Check
Which of the statements are correct about the reaction given below ?
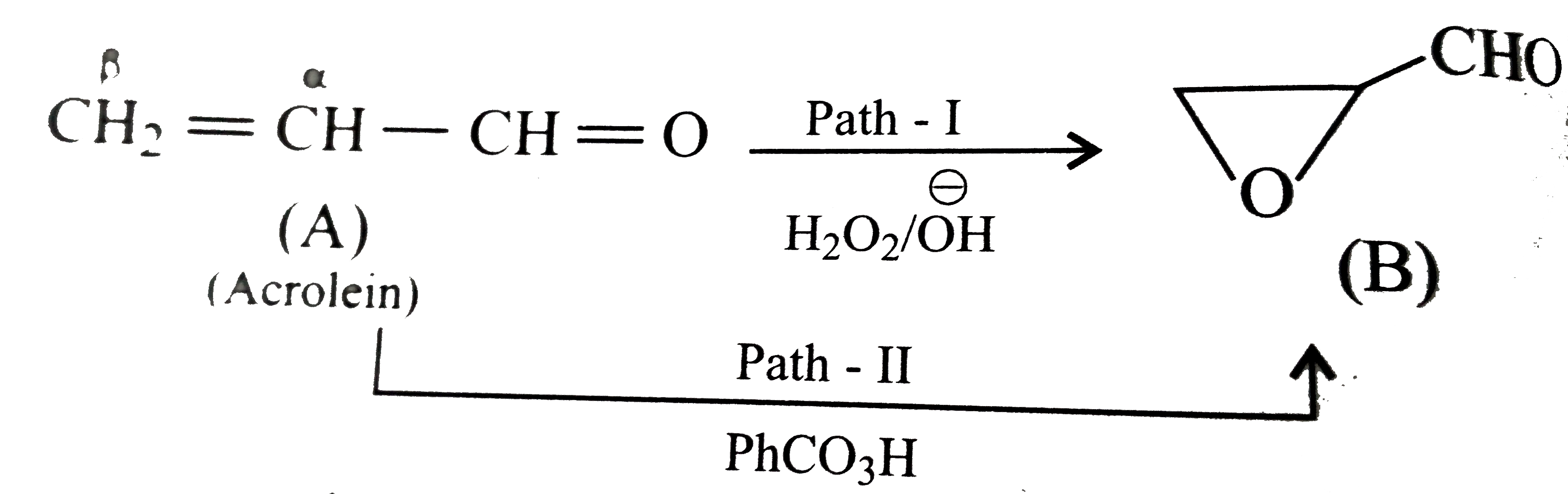 AFormation of (B) by Path I is much more faster than by Path II.BFormation of (B) by Path II is much more faster than by Path I.CFormation of (B) by both paths is equal.D(B) is formed by Path II only.
AFormation of (B) by Path I is much more faster than by Path II.BFormation of (B) by Path II is much more faster than by Path I.CFormation of (B) by both paths is equal.D(B) is formed by Path II only.Which of the following statements are correct about the reaction given below ?
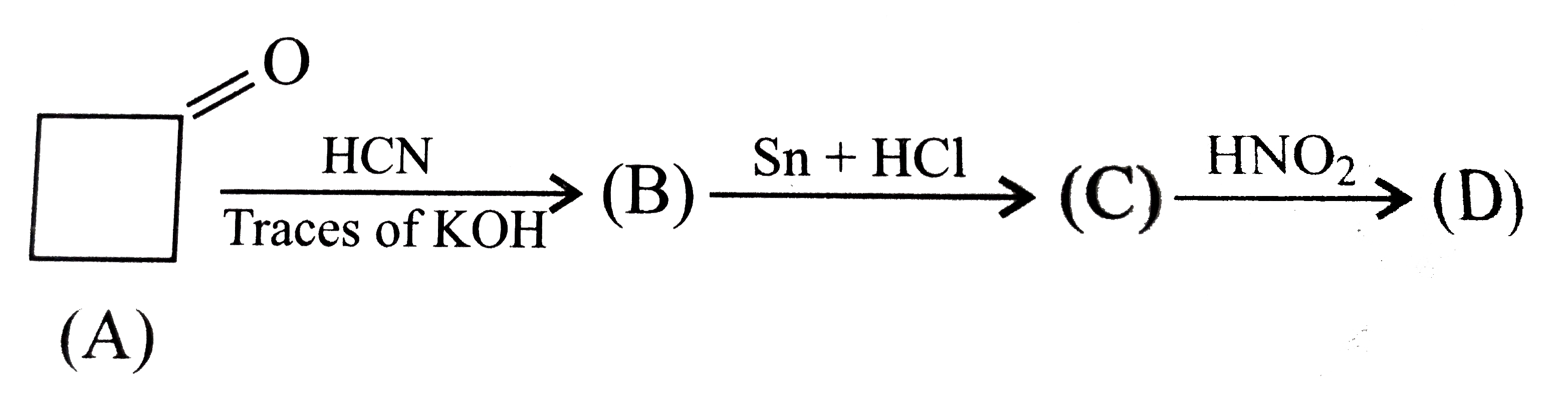 AIn the formation of (D) from (C ), ring expansion takes place.BThe product (D) is cyclopentanone.CThe product (D) is α,β-unsaturated cyclopentanoneDConversion of (B) to (C ) can also be carried out with LAH.
AIn the formation of (D) from (C ), ring expansion takes place.BThe product (D) is cyclopentanone.CThe product (D) is α,β-unsaturated cyclopentanoneDConversion of (B) to (C ) can also be carried out with LAH.Which of the statements are False about globalisation?
AAdvocates of globalisation argue that it will result in greater economic growthBCritics of globalisation argue that it will result in greater economic disparityCAdvocates of globalisation argue that it will result in cultural homogenisationDCritics of globalisation argue that it will result in cultural homogenisation
Which of the following statements about the gravitional constant are false ?
View SolutionWhich of these statements about decidua is false?
View SolutionWhich of the following statement is false about cabon ?
View SolutionWhich of the following statements about mutations is false?
View SolutionWhich of these statements about decidua is false?
View Solution

