Which is most stable carbocation?
A
Iso-propyl
B
Triphenylmethyl cation
C
Ethyl cation
D
π- propyl cation
Video Solution
Text Solution
The correct Answer is:B
Stability of carbonium ions. Ph3+C>Ph2+CH>Ph+CH2 tertiary alkyl > secondary alkyl > primary alkyl > methyl. More the number of phenyl groups, the greater the dispersal of positive charge and therefore, more the stability of carbonium ion is observed.
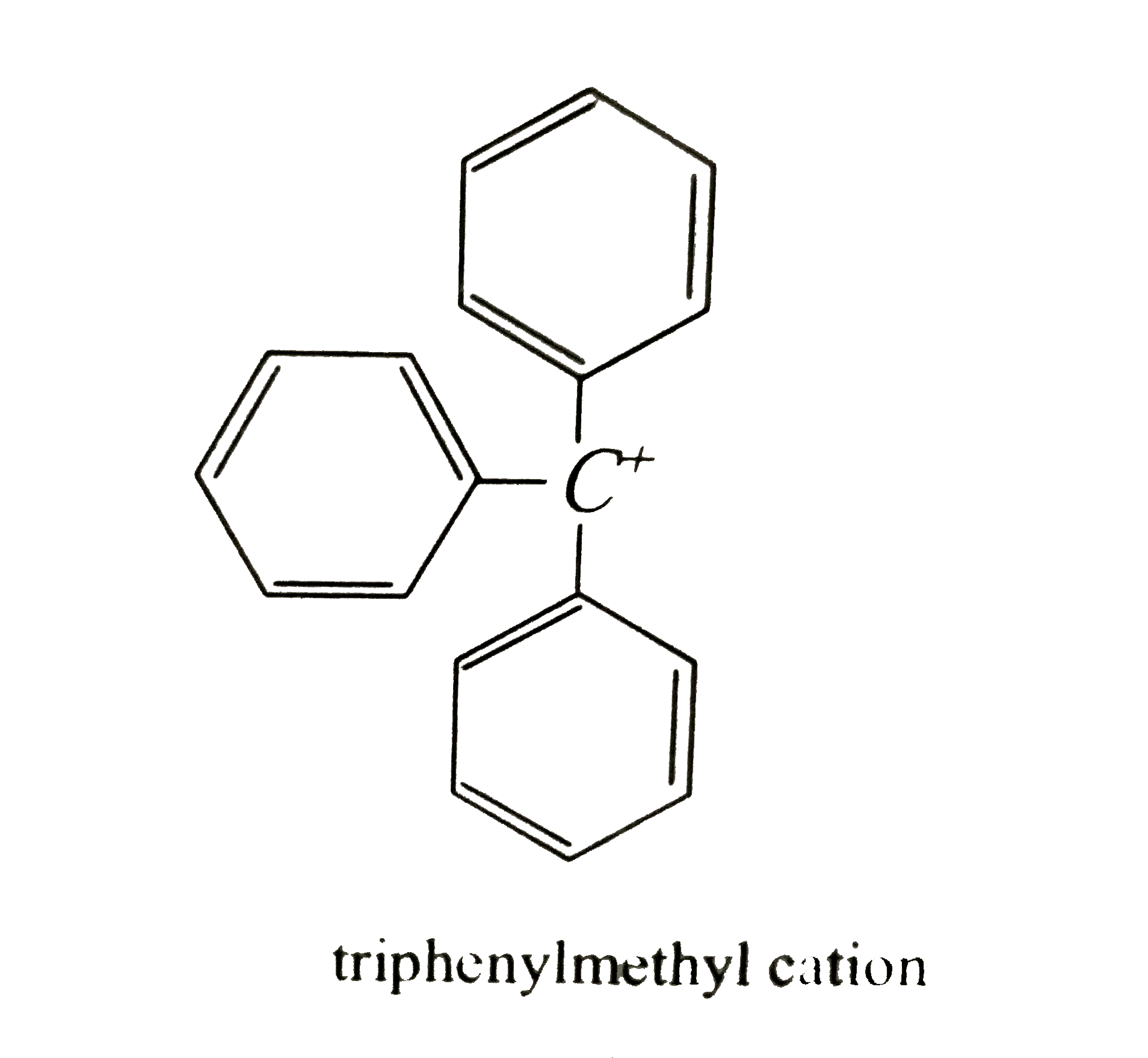
Dispersal of positive charge increases with the increase in the number of benzene ring.
|
Similar Questions
Knowledge Check
Which is the most stable carbocation ?
A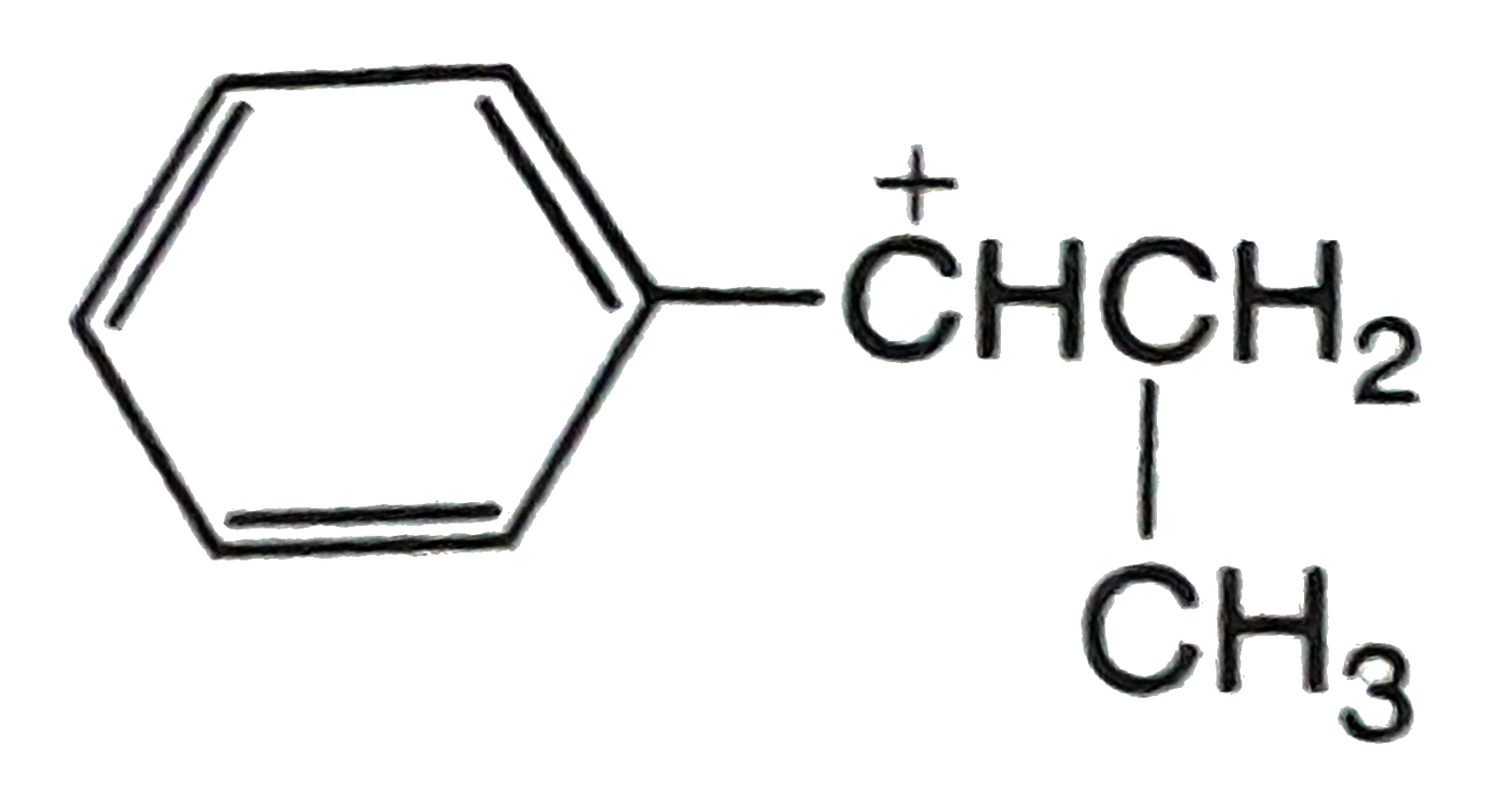 B
B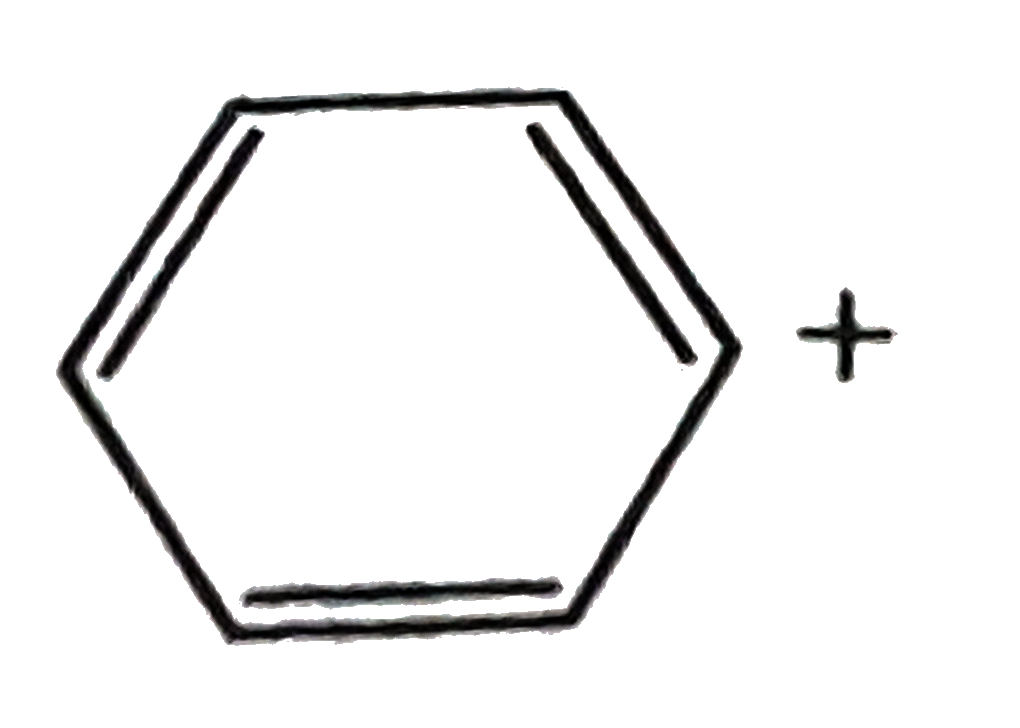 C
C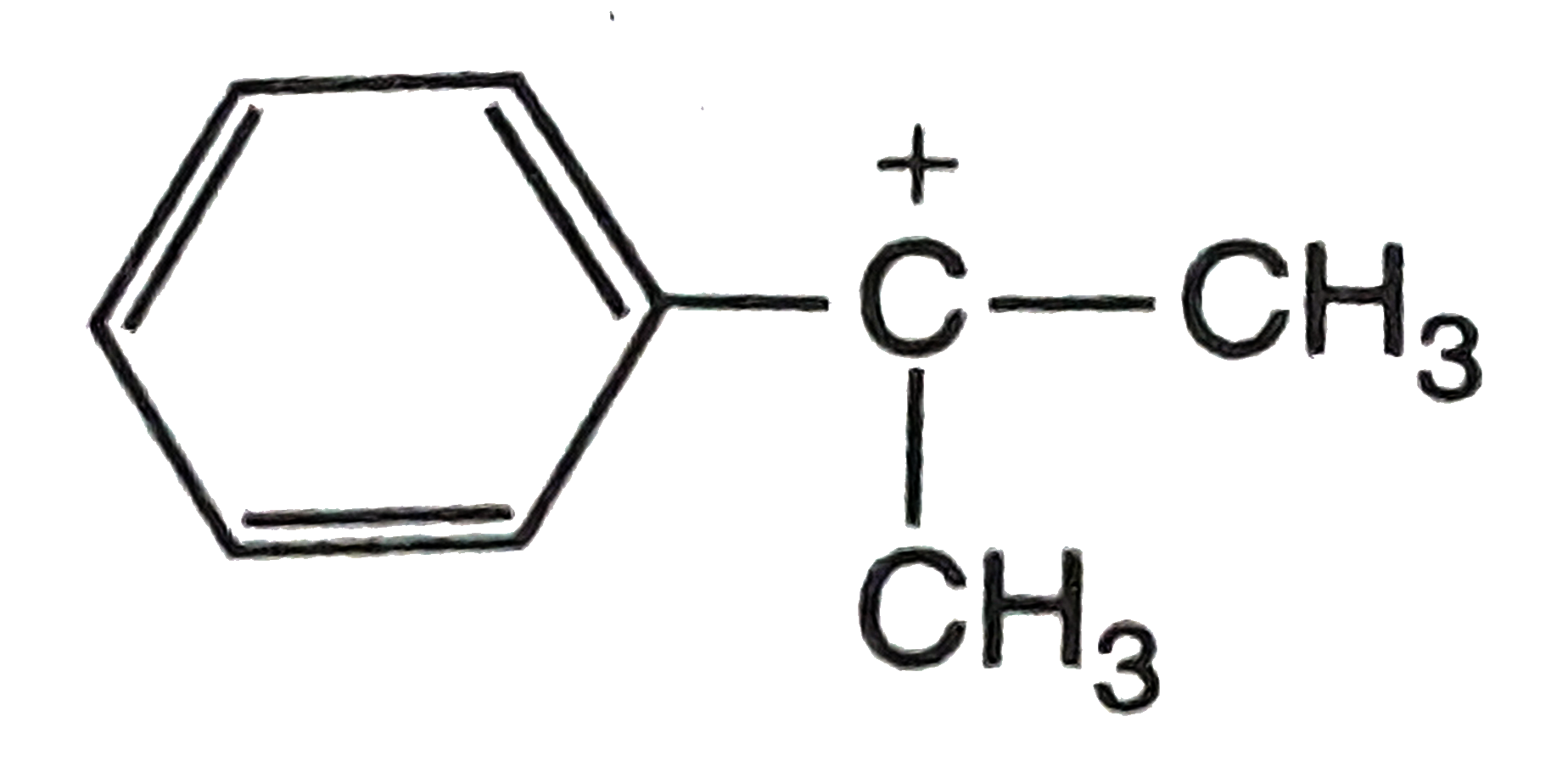 D
D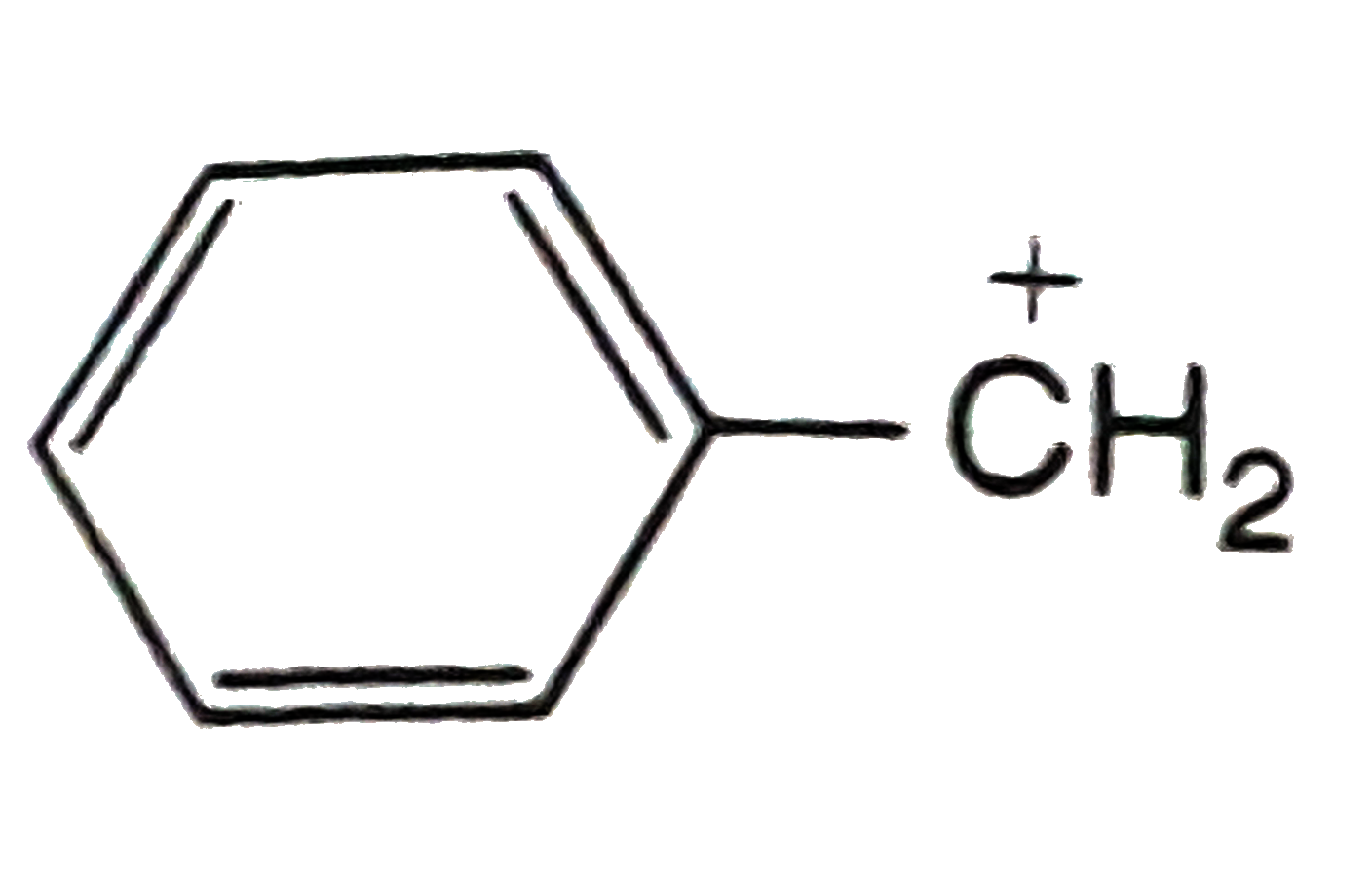
Which is the most stable carbocation?
A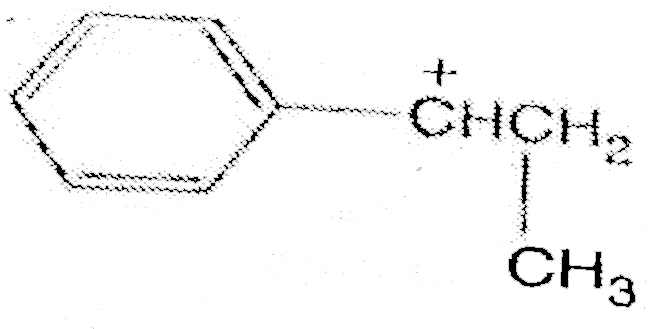 B
B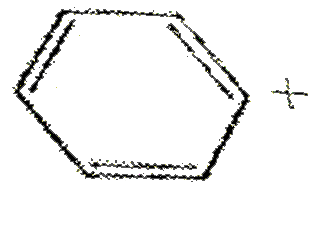 C
C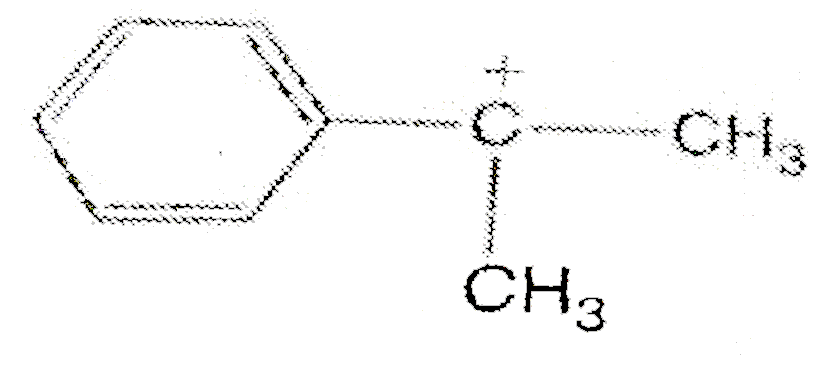 D
D
Which is most stable carbocation?
AIso-propylBTriphenylmethyl cationCEthyl cationDπ- propyl cation
Which one of the following is most stable carbocation ?
View SolutionWhich of the following is most stable carbocation?
View SolutionWhich of the following is most stable carbocation?
View SolutionWhich of the following is most stable carbocation?
View SolutionWhich one of the following is most stable carbocation ?
View Solution
