Which of the following is most basic
A
Benzamide
B
Butamine
C
Nitrobenzene
D
Benzene
Video Solution
Text Solution
The correct Answer is:B
Benzamide :
Does not show basic character because lone pair on nitrogen is delocalized with the carbonyl group which makes it has available for protonation.
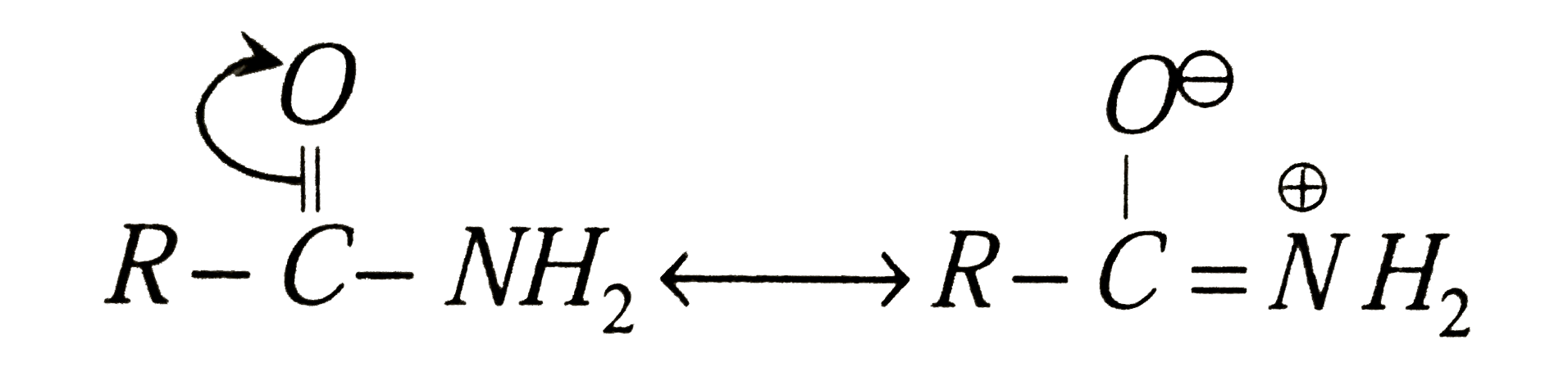
Butamine : CH3CH2CH2CH2NH2(aliphatic primary amine)
R→NH2
R- group due to +I effect intensify the electron density on nitrogen. Therefore highly basic in nature.
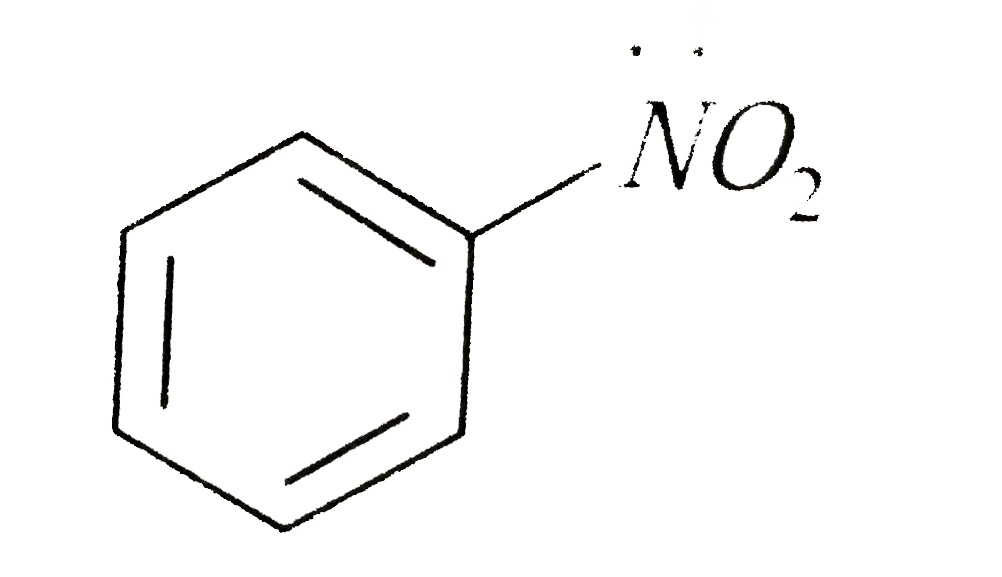
−NO2 group (-I) effect, therefore makes the ring electron deficient. Hence, acidic in nature.

|
Similar Questions
Knowledge Check
Which of the following si most basic:
A B
B C
C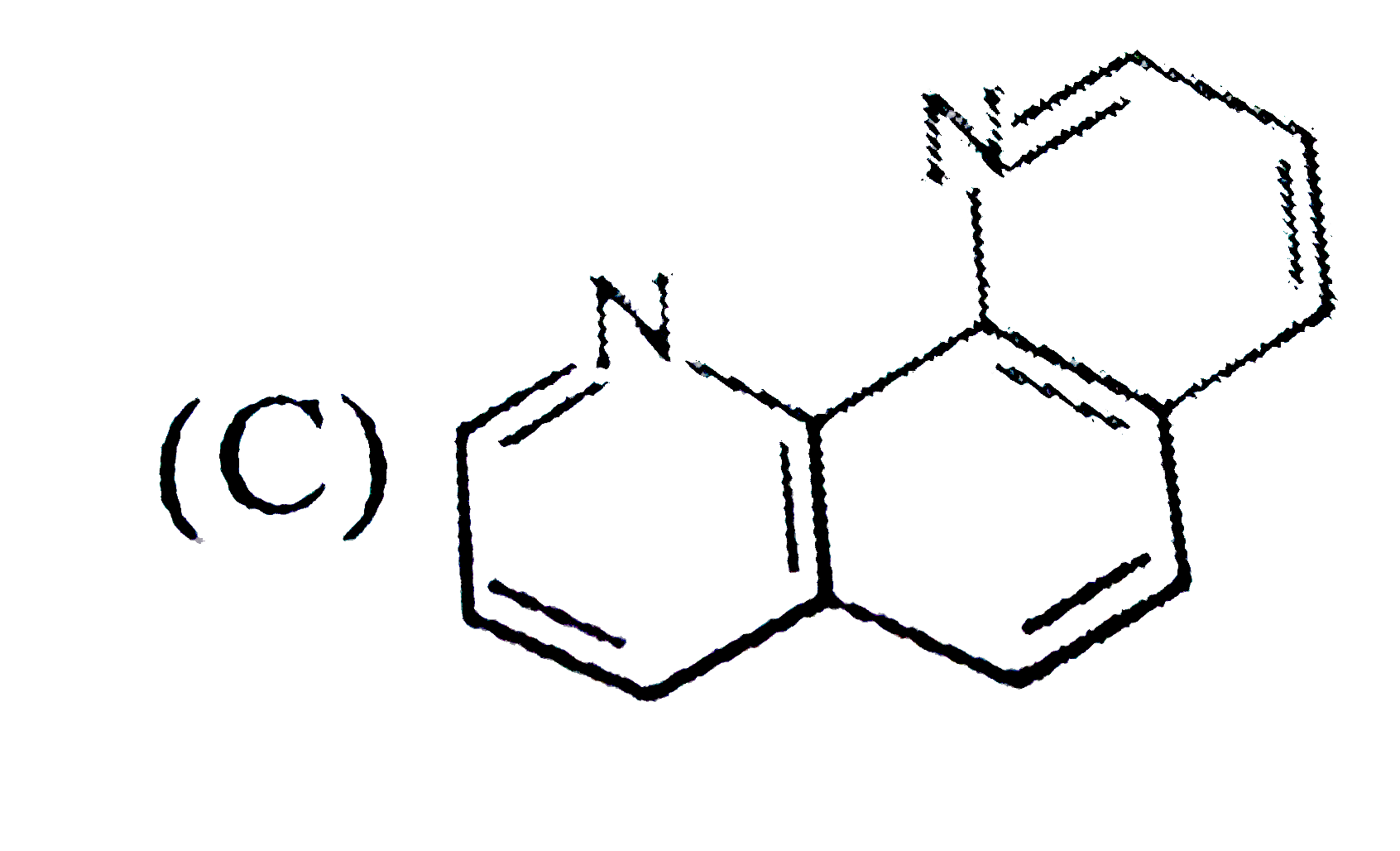 D
D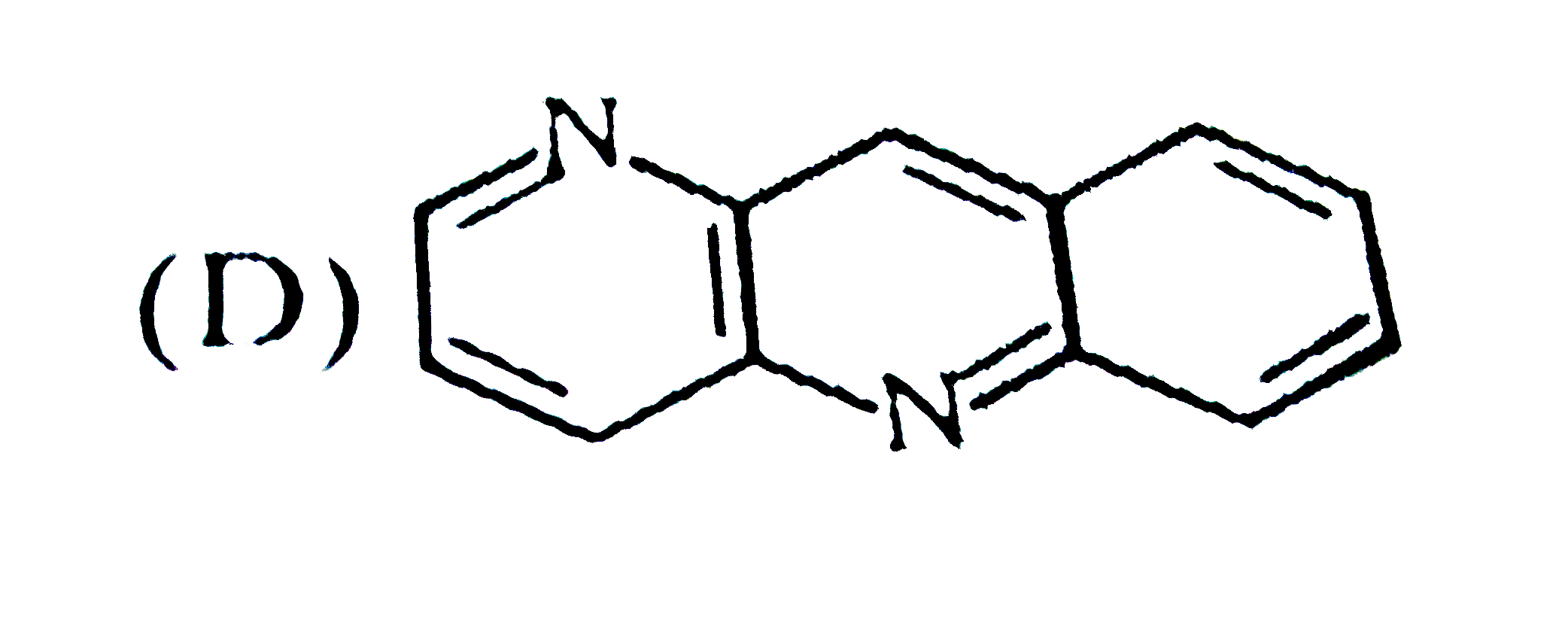
Which of the following is the most basic ?
Ap− phenylenediamineBm−phenylenediamineCanilineDp−bromoanilineWhich one of the following is most basic?
AF−BCl−CBr−DI−
Which of the following is the most basic in character
View SolutionWhich of the following is the most basic oxide /
View SolutionWhich one of the following is most basic?
View SolutionWhich one of the following is most basic
View SolutionWhich one of the following is most basic in gaseous phase?
View Solution

