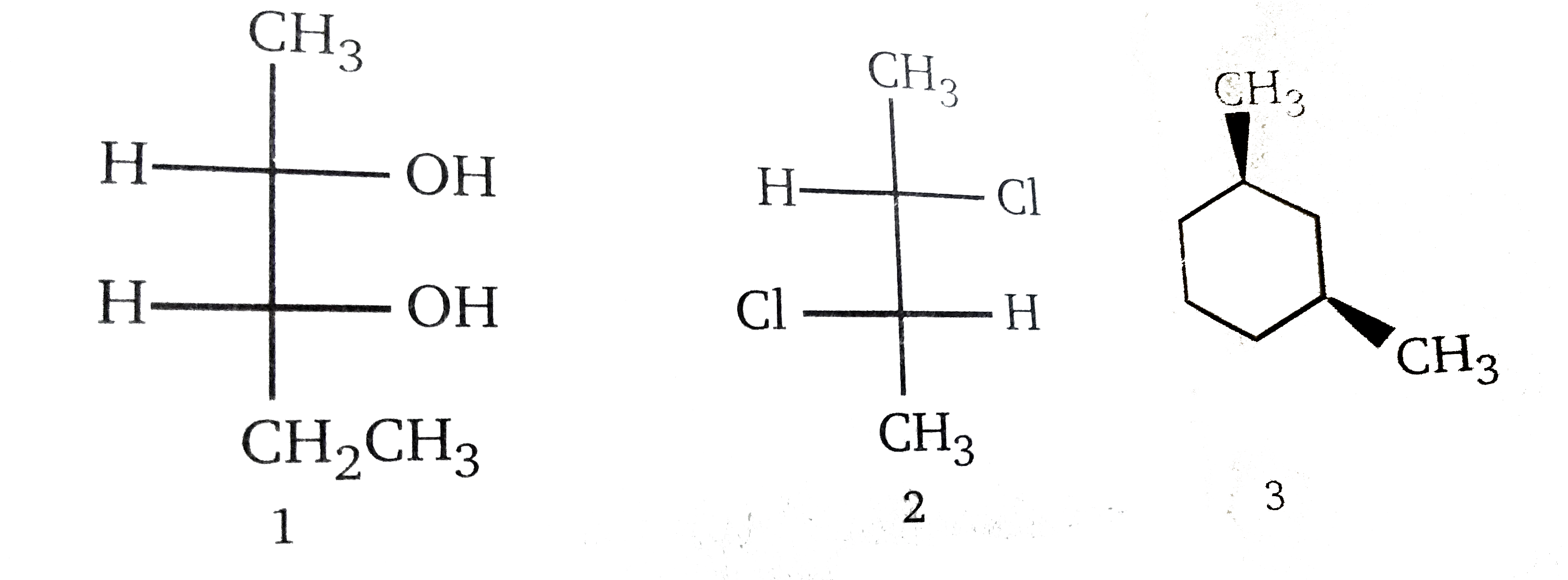
Which one of the following compound is capcabel of existing in a meso form
3,3-dibromopentane
4-bromo-2-pentanol
3-bromo-pentanol
2,4-dibromopentane
The correct Answer is:D
To determine which compound is capable of existing in a meso form, we need to understand the concept of meso compounds. A meso compound has two or more chiral centers but possesses an internal plane of symmetry, making it achiral overall.
1. Identify the Definition of Meso Compounds:
- A meso compound must have at least two chiral centers and an internal plane of symmetry.
2. Examine Each Compound:
- Option 1: 3,3-Dibromopentane:
- Check for chiral centers: There are no chiral centers in this compound.
- Conclusion: Not a meso compound.
- Option 2: 4-Bromo-2-pentanol:
- Check for chiral centers: This compound has two chiral centers.
- Check for symmetry: There is no plane of symmetry present.
- Conclusion: Not a meso compound.
- Option 3: 3-Bromo-3-pentanol:
- Check for chiral centers: This compound has only one chiral center.
- Conclusion: Not a meso compound (meso compounds cannot have only one chiral center).
- Option 4: 2,4-Dibromopentane:
- Check for chiral centers: This compound has two chiral centers.
- Check for symmetry: There is an internal plane of symmetry.
- Conclusion: This compound is a meso compound.
3. Final Answer:
- The compound capable of existing in a meso form is Option 4: 2,4-Dibromopentane.
- A meso compound must have at least two chiral centers and an internal plane of symmetry.
- Option 1: 3,3-Dibromopentane:
- Check for chiral centers: There are no chiral centers in this compound.
- Conclusion: Not a meso compound.
- Check for chiral centers: This compound has two chiral centers.
- Check for symmetry: There is no plane of symmetry present.
- Conclusion: Not a meso compound.
- Check for chiral centers: This compound has only one chiral center.
- Conclusion: Not a meso compound (meso compounds cannot have only one chiral center).
- Check for chiral centers: This compound has two chiral centers.
- Check for symmetry: There is an internal plane of symmetry.
- Conclusion: This compound is a meso compound.
- The compound capable of existing in a meso form is Option 4: 2,4-Dibromopentane.
Similar Questions
Knowledge Check
Which one of the following compounds is capable of existing in a meso form?
A3,3-DibromopentaneB4-Bromo-2-pentanolC3-Bromo-2-pentanolD2,3-DibromopentaneWhich of the following compounds exists ?
AKHCl2BKHF2CKHBr2DKHI2Which one of the following compounds does not exist?
ANCl5BAsF5CSbCl5DPF5