Which of the following orders regarding relative stability of free radicals is correct?
A
3∘<2∘<1∘
B
3∘>2∘>1∘
C
1∘<2∘>3∘
D
3∘>2∘<1∘
Video Solution
Text Solution
The correct Answer is:B
Due to the increasing no. of hyperconjugative structures free radical stabilise as following 3∘>2∘>1∘.
|
Similar Questions
Knowledge Check
Which of the following order regarding the stability of intemediates is not correctly arranged?
AF2..C<Cl2..C>Br2..>I2..CB(C6H5)3..C>(C6H5)2.CH>CH2=CH−.CH2CR - C ≡¯¯¯C<R2C=¯¯¯CH<R3C−¯¯¯CH2D(C6H5)3+C<(C6H5)2C+>CH+2−CH=CH2Which of the following orders are correct regarding stability?
A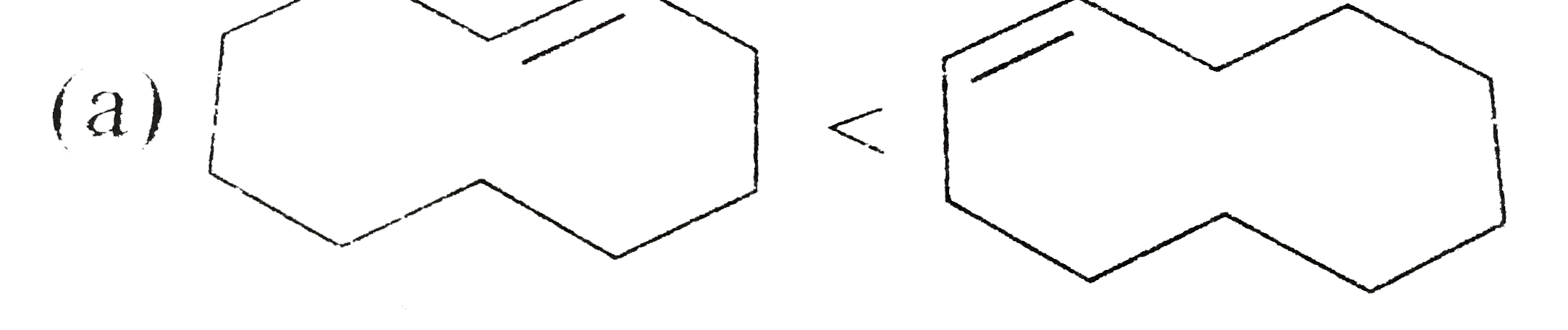 B
B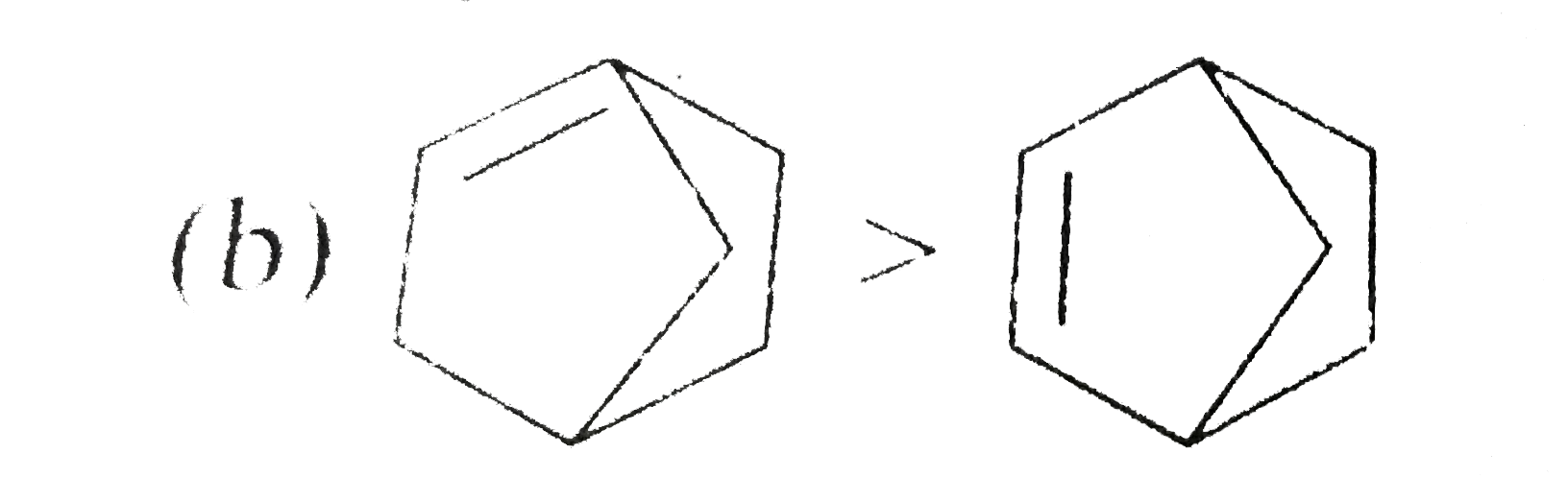 C
C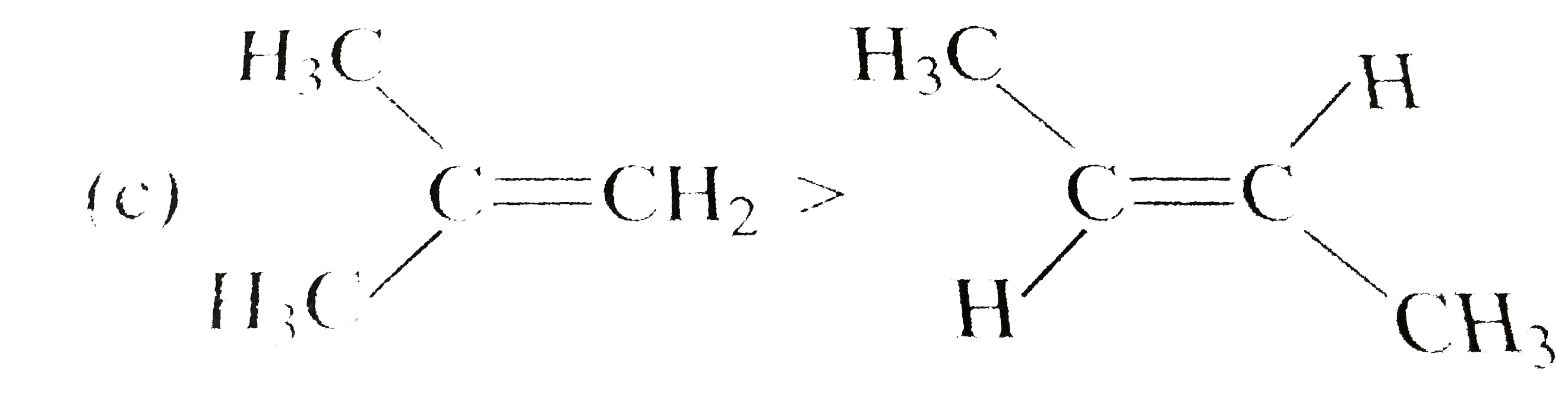 D
D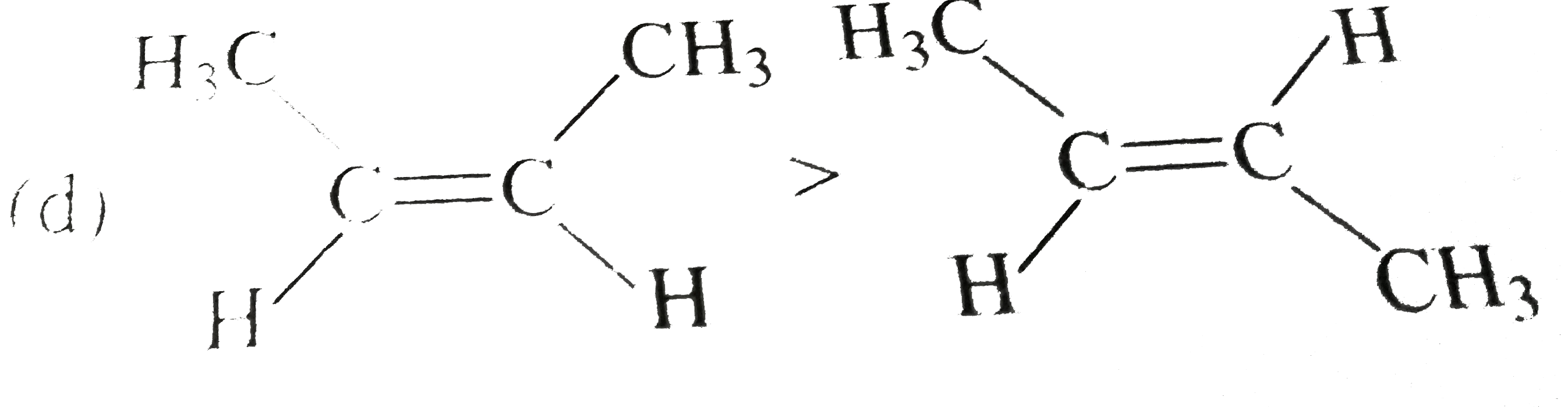
Which of the following is the correct order of stability of free radicals ?
Abenzyl > allyl >2o−>1o−Ballyl > benzyl >2o−>1o−Callyl >2o−>1o− benzylDbenzyl >2o−>1o− allyl
Which of the following is free radical?
View SolutionThe order of stability of free radical is-
View SolutionWhich of the following orders regarding acidic strength is correct
View SolutionWhich of the following order is correct regarding the relative basicity of amines
View SolutionWhich of the following orders regarding acidic strength is correct.
View Solution
