P and Q are isomers of dicarboxylic acid C4H4O4. Bothdecolorize Br2/H2O. On heating, P forms the cyclic anhydride. Upon treatment with dilute alkaline KMnO4,P as well as Q could produce one or more than one forms S,T and U.
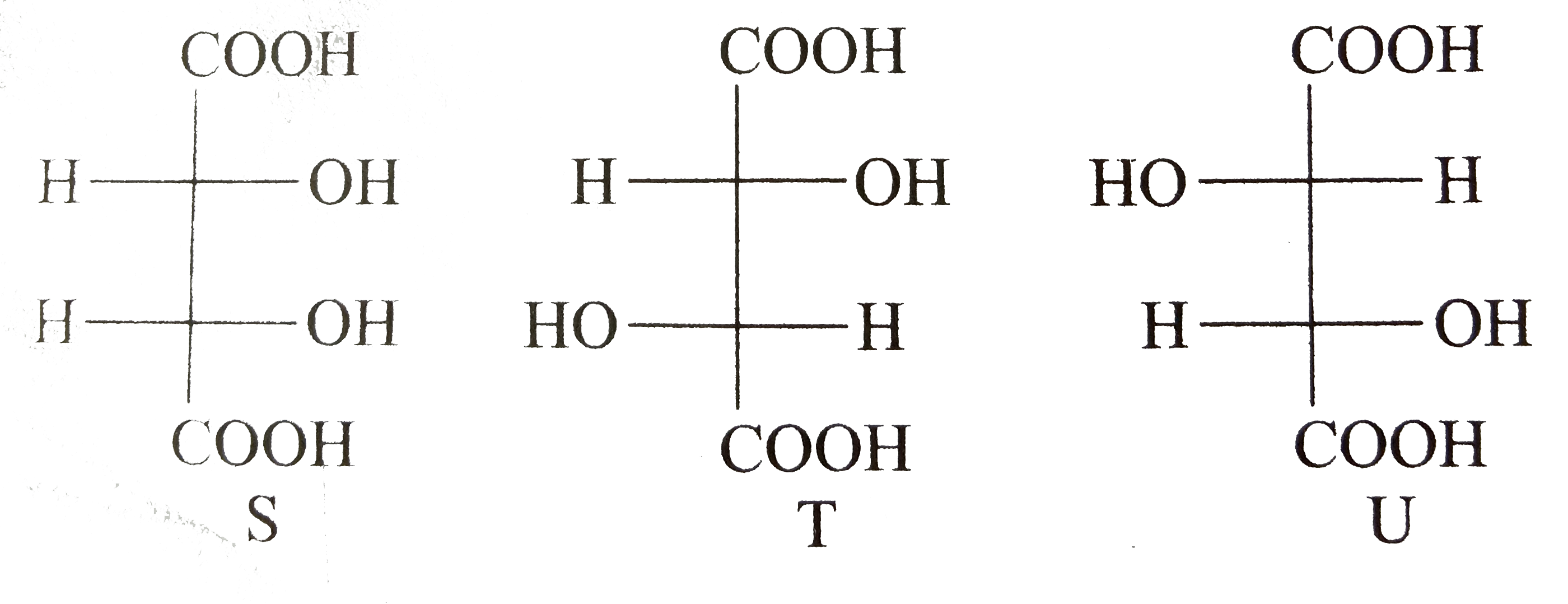 Compounds formed from P and Q are, respectively
Compounds formed from P and Q are, respectively
Optically active S and optically active pair (T, U)
Optically inactive S and optically inactive pair (T, U)
Optically active pair (T, U) and optically active S
Optically inactive pair (T, U) and optically inactive S
The correct Answer is:A
Step by step video & image solution for P and Q are isomers of dicarboxylic acid C_(4)H_(4)O_(4). Bothdecolorize Br_(2)//H_(2)O. On heating, P forms the cyclic anhydride. Upon treatment with dilute alkaline KMnO_(4),P as well as Q could produce one or more than one forms S,T and U. Compounds formed from P and Q are, respectively by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Which pure compounds form intermolecular hydrogen bonds?
(P) HF
(Q) H2S
(R) CH4
View SolutionFor the given redox reaction x KMnO_(4) +y H_(2)O_(2) +z H_(2)SO_(4) rarr p MnSO_(4) +10 O_(2(d)) + q K_(2)SO_(4) +r H_(2)O Calculate value of (x+z-p)
View Solution[P] overset(Br_(2))to C_(2)H_(4)Br_(2) underset(NH_(3))overset(NaNH_(2))to [Q] underset(Hg^(2+), Delta)overset(20% H_(2)SO_(4))to [R] overset(Zn-Hg // HCl)to [S] The species P, Q, R and S respectively are
View SolutionP and Q are isomers C4H4O4 of dicarboxylic acid both decolorise Br2/H2O. On heating P forms the cyclic anhydride. Upon treatment with dilute alkaline KMnO4, P as well as Q could produce one or more than one form S, T and U
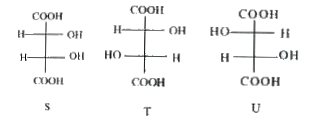
Compounds formed from P and Q are respectively
View Solution[P] on treatment with H_(2)CrO_(4) in acetone produced [Q]. [Q] compound upon heating give rise to Acetophenone. The compound [P] is:
View SolutionIf p,q>0,p+q=2 and p4+q4=272, then p and q are roots of
View SolutionThe pq form of 4.(¯7) is _______
View Solutiona. Isomers (A) and (B), C_(10)H_(12)O , are isolated form the oil of bay leaf. They are insoluble in aq. NaOH and decolourise Br_(2)//CCI_(4) and KMnO_(4) . Each is vigorously oxidised to p-anisic acid, (p-MeOC_(6)H_(4)COOH) . Ozonolysis (A) and (B). Identify A and B
View Solution
