Which of the following aromatic acids is most acidic?
A
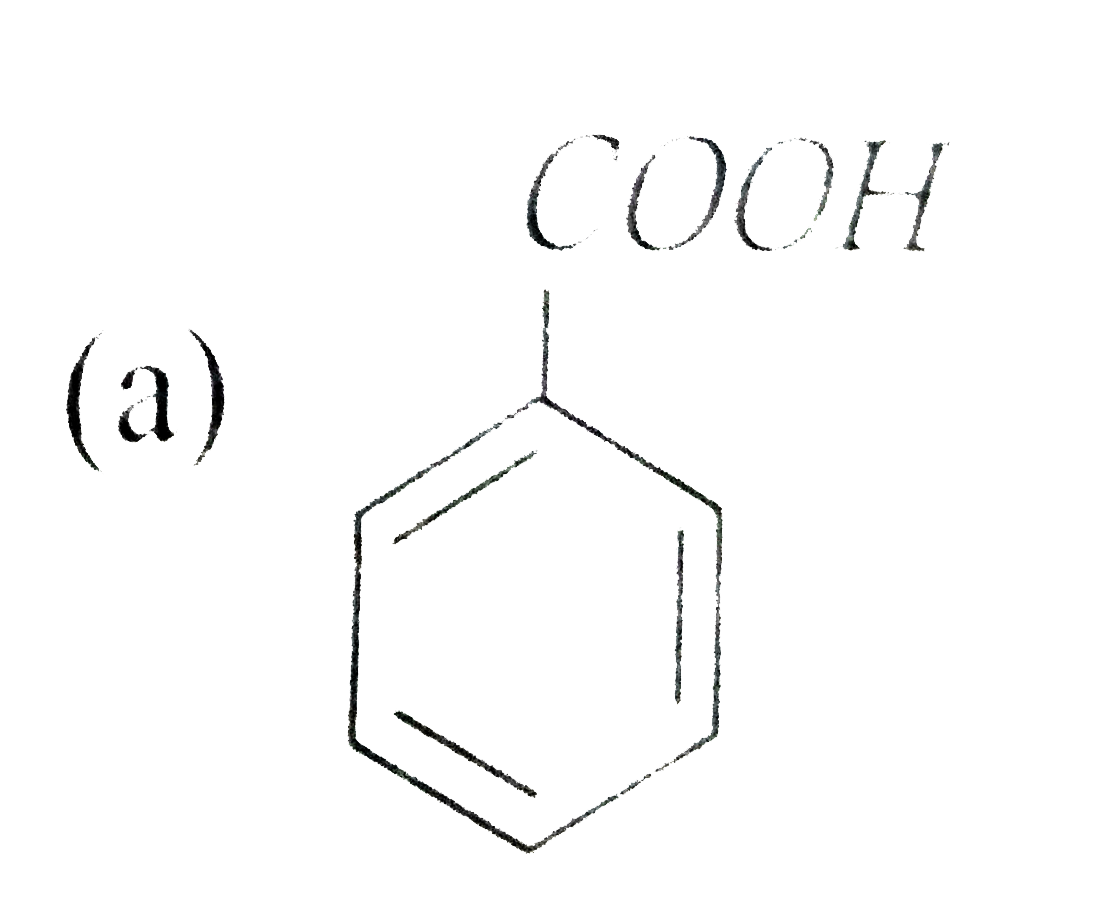
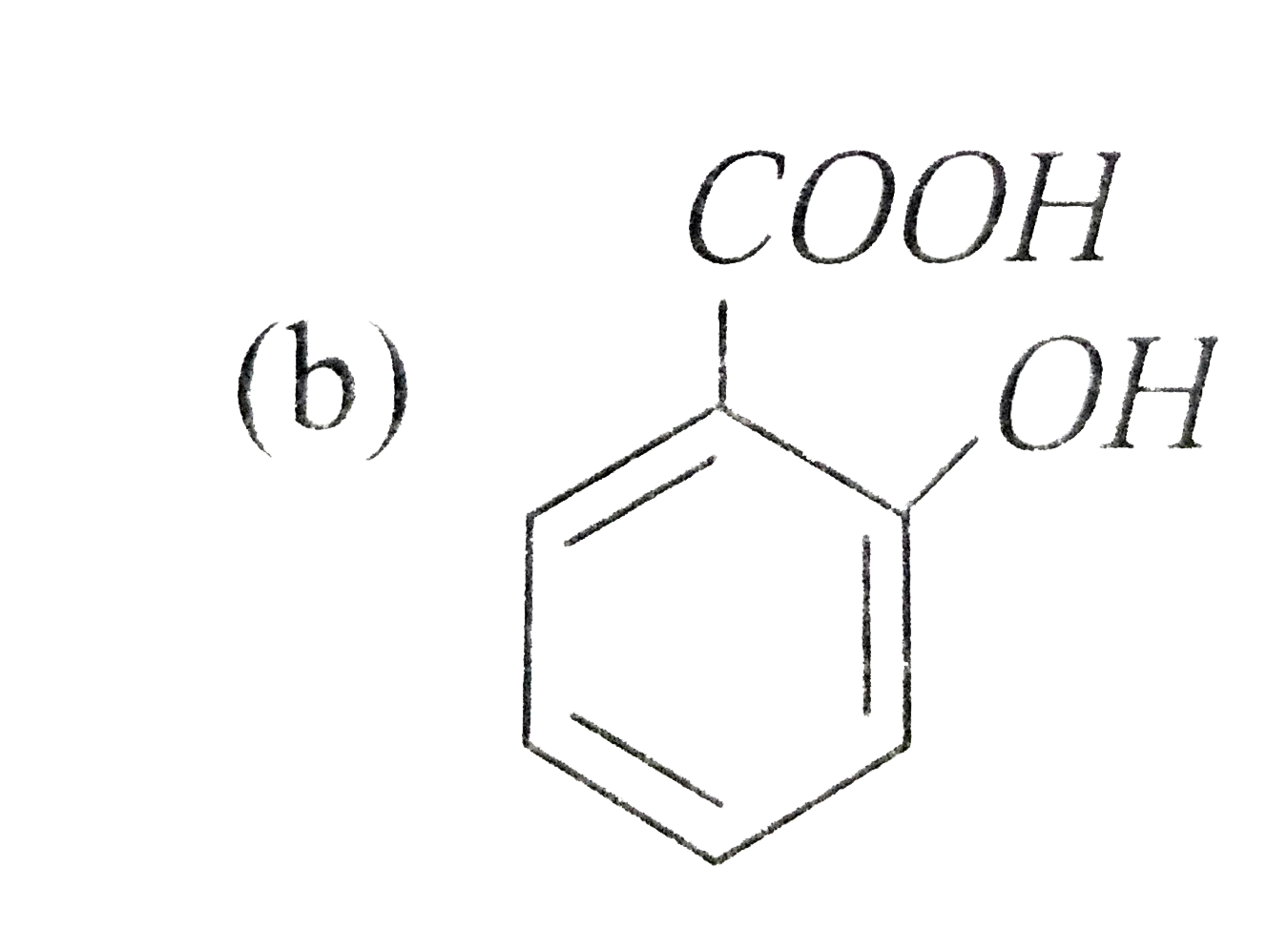
B
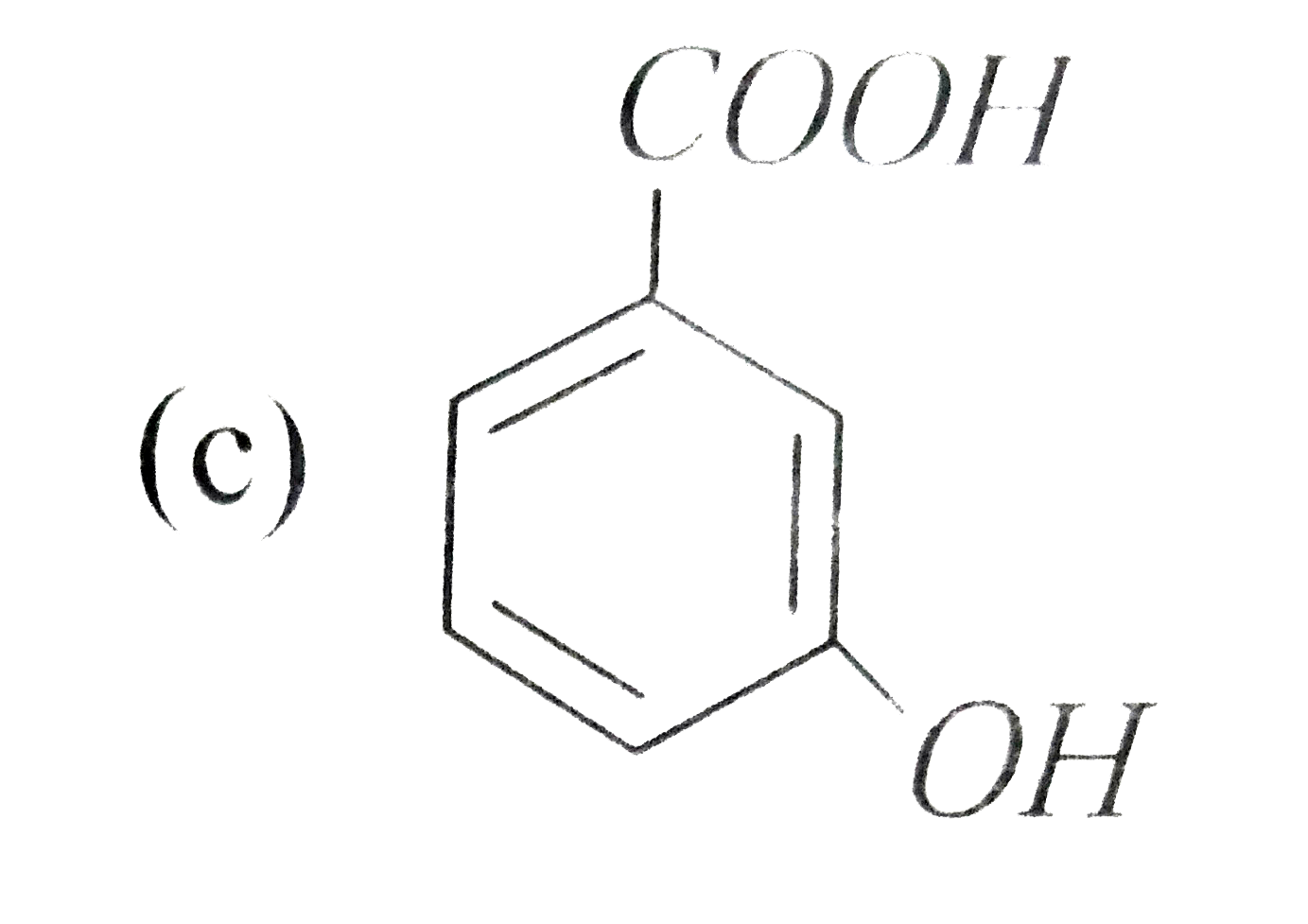
C
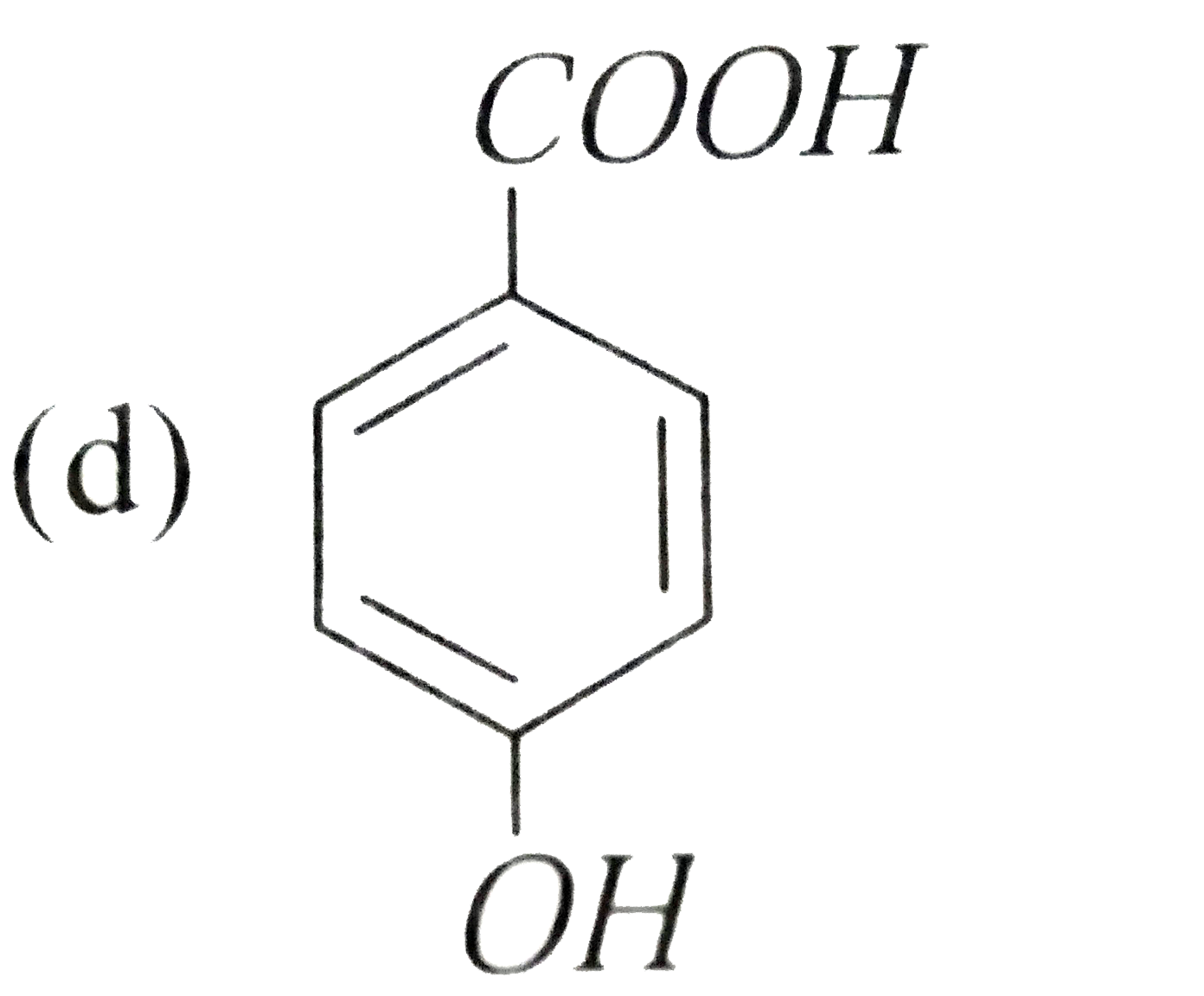
D
Video Solution
Text Solution
The correct Answer is:B
it is due to ortho-effect i.e., ortho substituted acid is stronger. Benzoic acid is a resonance hybrid, and so the carbonyl group is coplanar with the ring. An o-substituent tends to prevent this coplanarity. The resonance is diminished (or prevented), and so the O atom of the -OH group has a greater positive charge, resulting in increased acid-strength.
|
Similar Questions
Knowledge Check
Which of the following aromatic acid is most acidic (lowest pKa value)?
A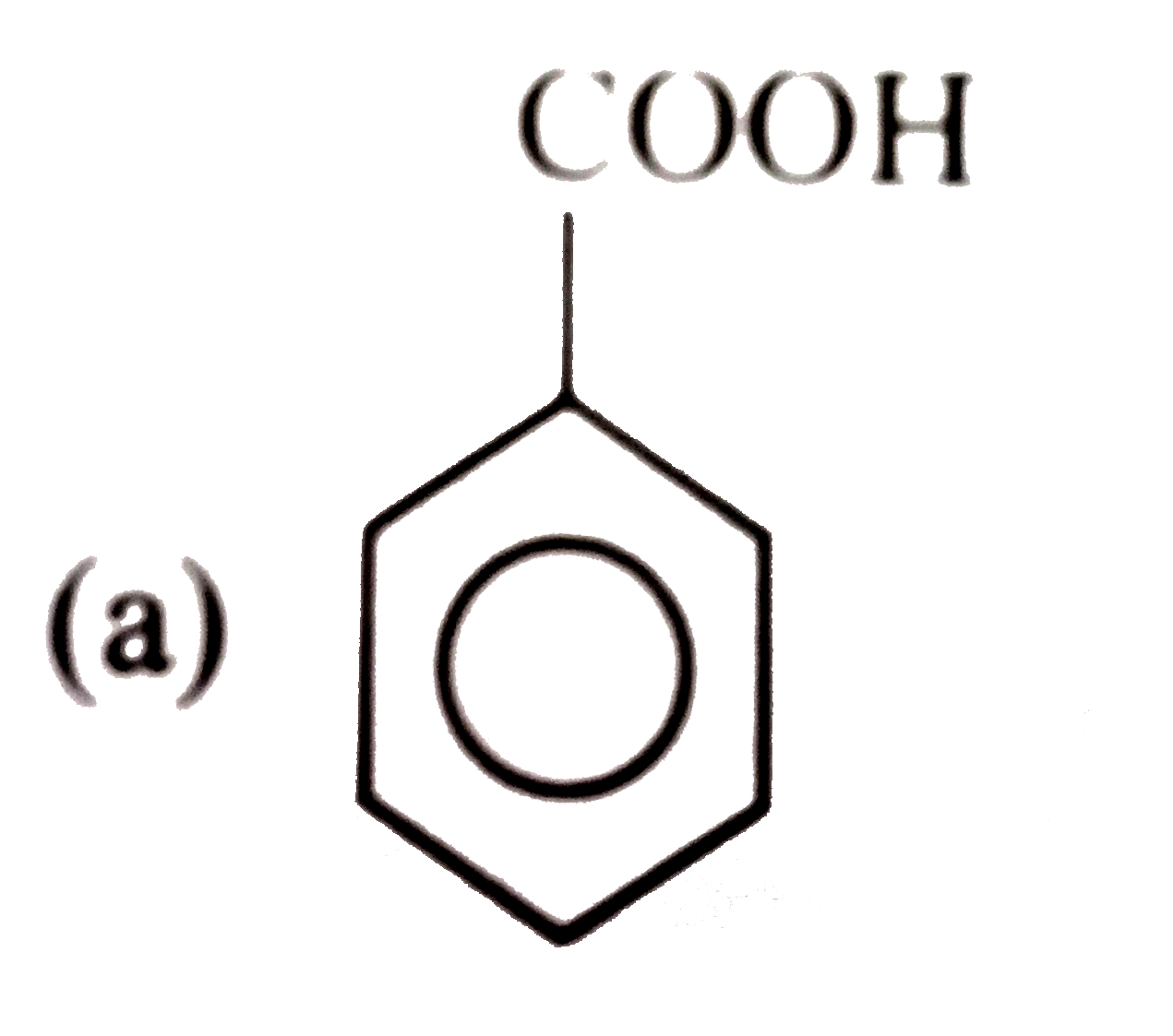 B
B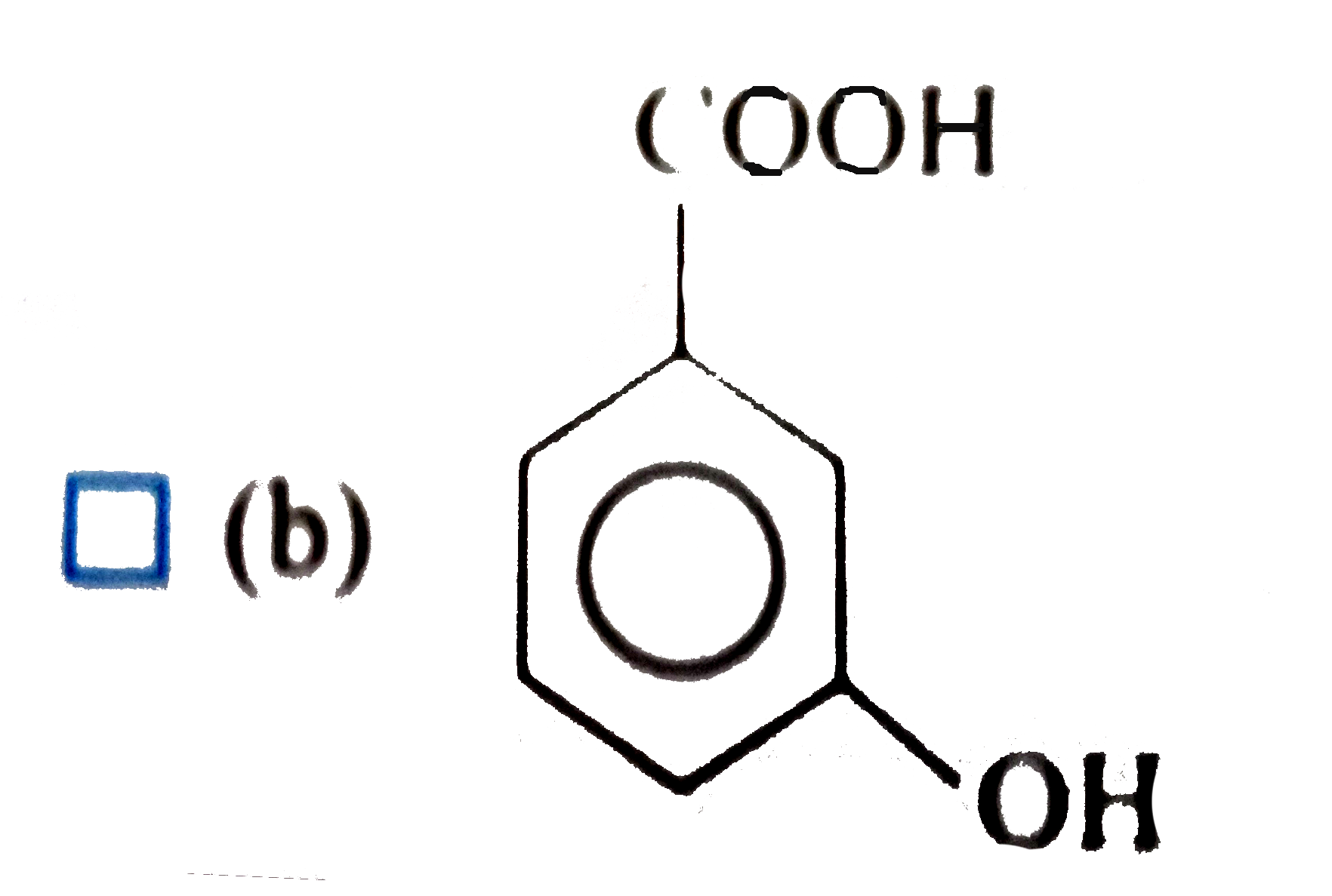 C
C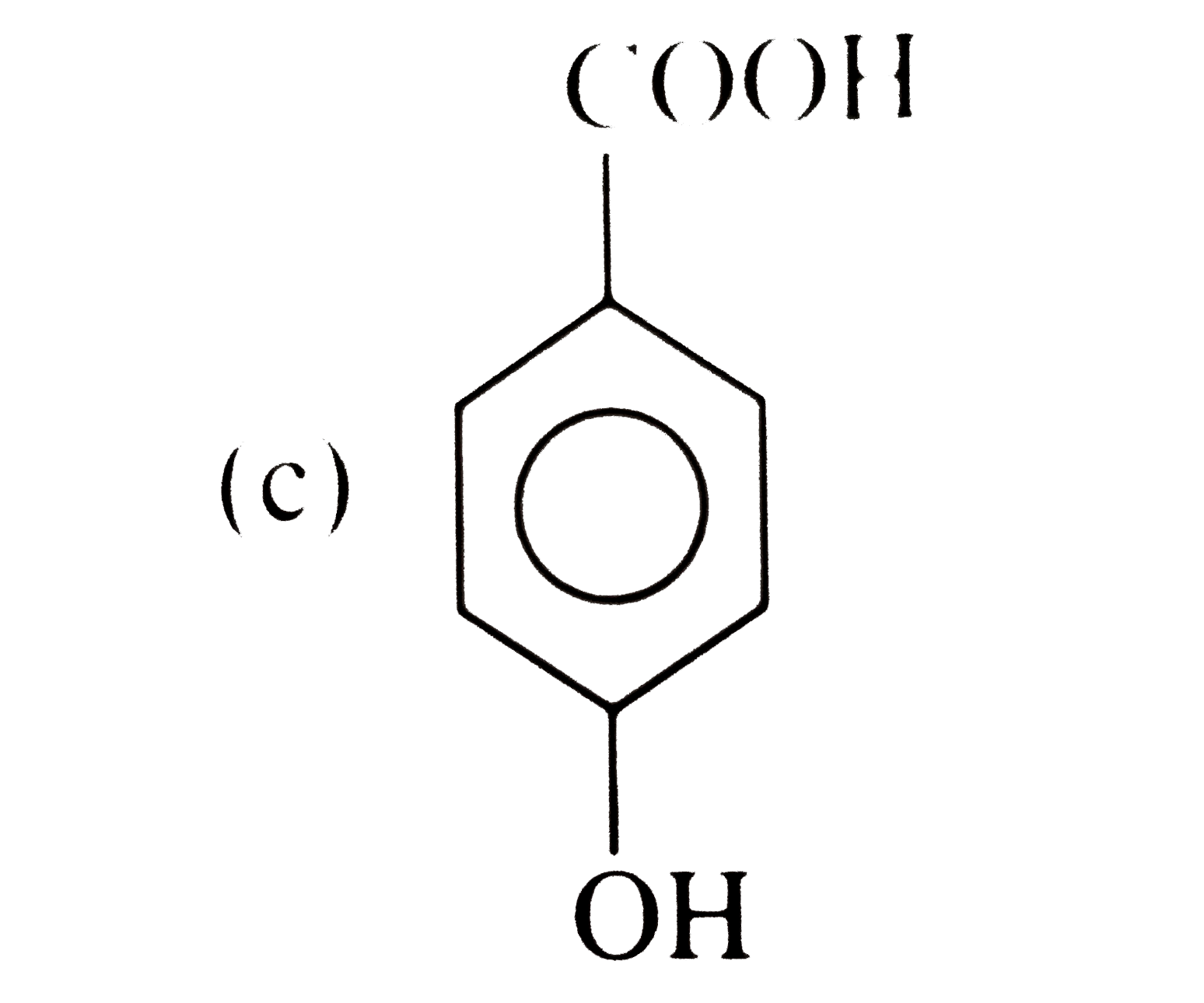 D
D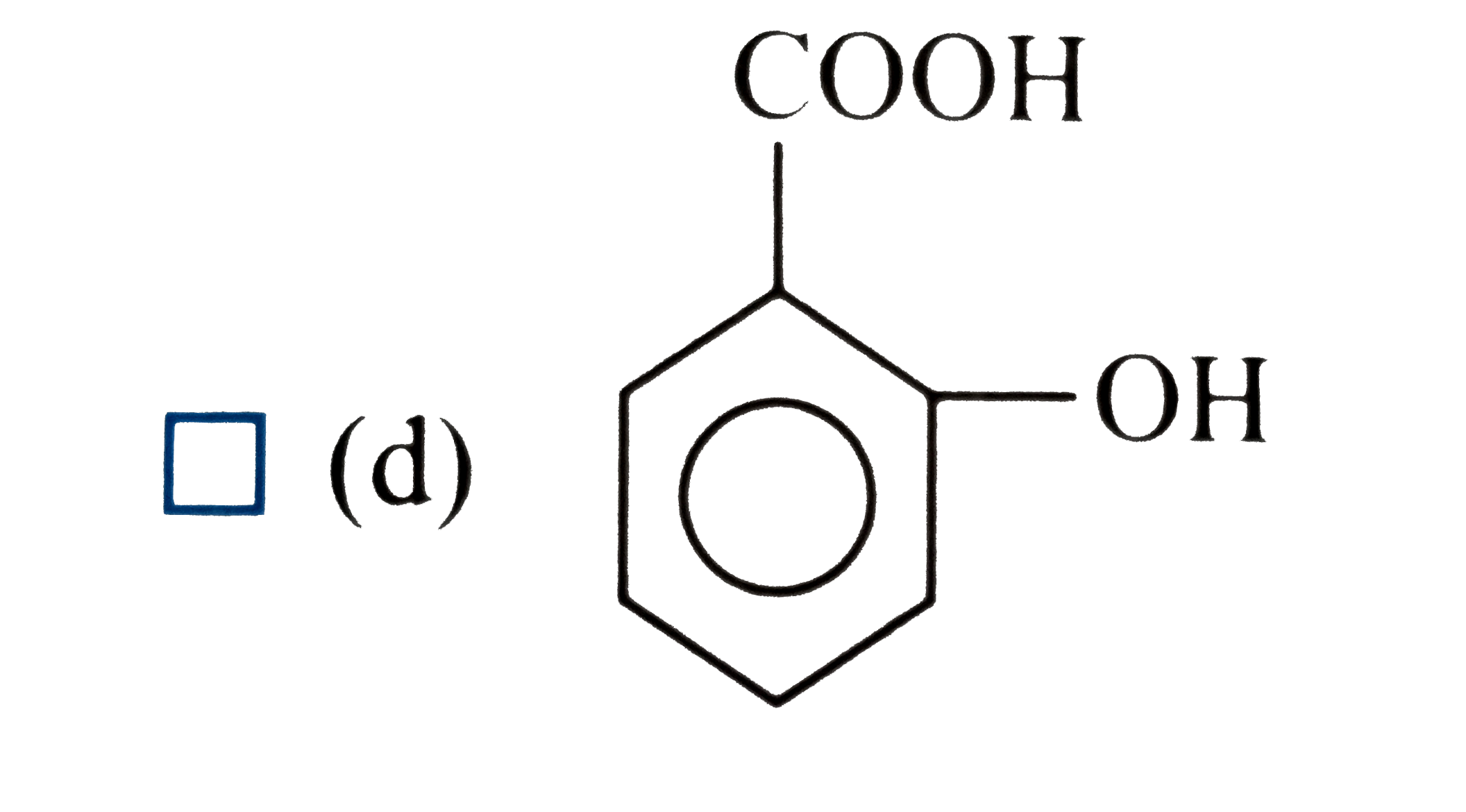
Which of the following carboxylic acids is most acidic in character-
Ao-methyl banzoic acidBm-methyl benzoic acidCp-methyl benzoic acidDBenzoic acidWhich of the following aqueous acid is most acidic?
AHCIBHFCHIDHBr
Which of the following is most acidic?
View SolutionWhich of the following compounds is the most acidic ?
View SolutionWhich of the following compounds is the most acidic ?
View SolutionWhich of the following compounds is the most acidic
View SolutionWhich of the following hydrides is the most acidic?
View Solution
