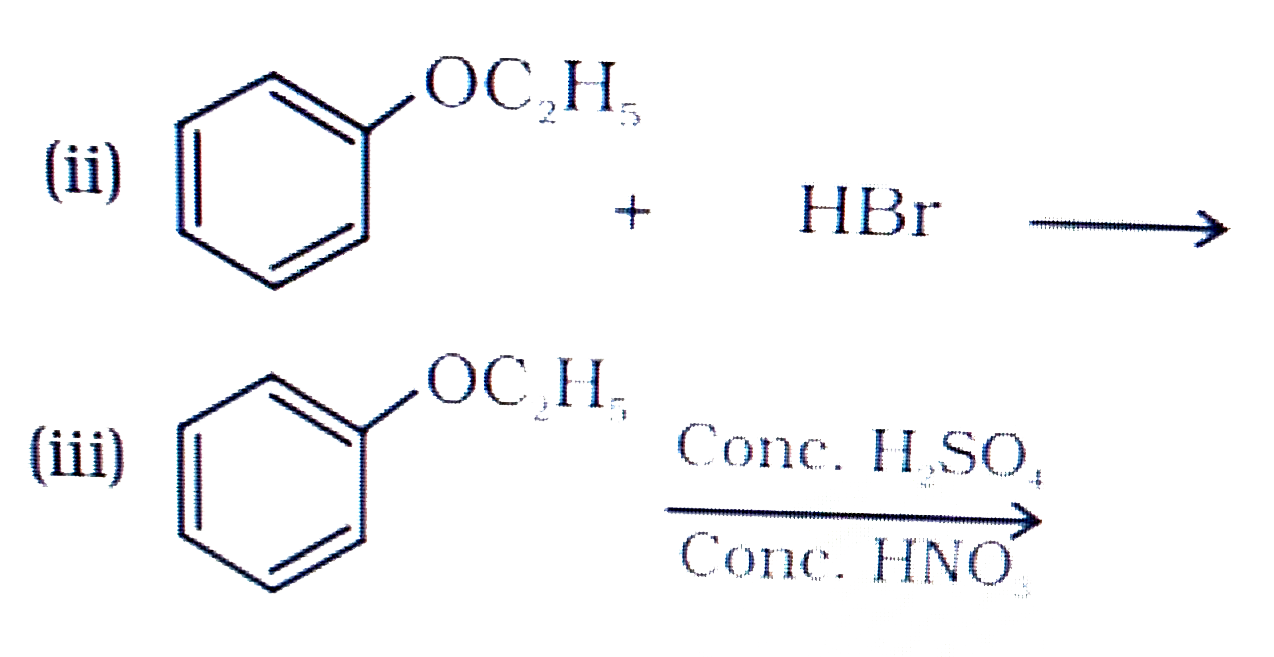The product X in the following reaction is (CH3CO)2O+C2H5OH→CH3COOH+X
C2H5COOH
CH3COOCH3
CH3COOC2H5
CH3COCH3
The correct Answer is:C
To solve the reaction (CH₃CO)₂O+C₂H₅OH→CH₃COOH+X, we need to identify the product X formed when acetic anhydride reacts with ethanol.
1. Identify the Reactants:
- The reactants are acetic anhydride (CH₃CO)₂O and ethanol C₂H₅OH.
2. Understand the Reaction Mechanism:
- Acetic anhydride can react with alcohols through a nucleophilic acyl substitution mechanism. In this case, ethanol acts as a nucleophile.
3. Nucleophilic Attack:
- The oxygen atom of the ethanol molecule attacks the carbonyl carbon of one of the acetic anhydride's carbonyl groups. This forms a tetrahedral intermediate.
4. Formation of Acetic Acid:
- The tetrahedral intermediate collapses, leading to the formation of acetic acid CH₃COOH and the other product.
5. Identify the Other Product (X):
- The other product formed from the reaction is ethyl acetate CH₃COOCH₂CH₃. This is because the remaining acyl group from acetic anhydride combines with the ethanol.
6. Final Products:
- The final products of the reaction are acetic acid CH₃COOH and ethyl acetate CH₃COOCH₂CH₃.
Conclusion:
The product X in the reaction is ethyl acetate, which can be represented as CH₃COOCH₂CH₃.
- The reactants are acetic anhydride (CH₃CO)₂O and ethanol C₂H₅OH.
- Acetic anhydride can react with alcohols through a nucleophilic acyl substitution mechanism. In this case, ethanol acts as a nucleophile.
- The oxygen atom of the ethanol molecule attacks the carbonyl carbon of one of the acetic anhydride's carbonyl groups. This forms a tetrahedral intermediate.
- The tetrahedral intermediate collapses, leading to the formation of acetic acid CH₃COOH and the other product.
- The other product formed from the reaction is ethyl acetate CH₃COOCH₂CH₃. This is because the remaining acyl group from acetic anhydride combines with the ethanol.
- The final products of the reaction are acetic acid CH₃COOH and ethyl acetate CH₃COOCH₂CH₃.
The product X in the reaction is ethyl acetate, which can be represented as CH₃COOCH₂CH₃.
Similar Questions
Knowledge Check
The product "A" in the following reaction is :CH3−CO−CH2COOC2H5NaOH−−−−−−→H2OA
ACH3COOHBC2H5OHCCH3COCH3DC2H5CHOThe product of following reaction is-
C2H5O−+CH3CH2I→AC6H5OC2H5BC2H5OC2H5CC6H5O−+CH3CH2IDC6H5IEnd product in the following reactions
(CH3)3C−CHO+CH3CH2CHOdiluteOH−−−−−−−→AH+−−−→−H2OBNaBH4−−−−→C is _________.A(CH3)3C−C∣CH3=CHCH2CH2OHB(CH3)3C−CH2−CH2OHC(CH3)3C−CH2CH2CH2OHD(CH3)3C−CH=CCH3−CH2OH
Predict the products of the following reactions :
(i) CH3−CH2−CH2−O−CH3+HBr→
(ii)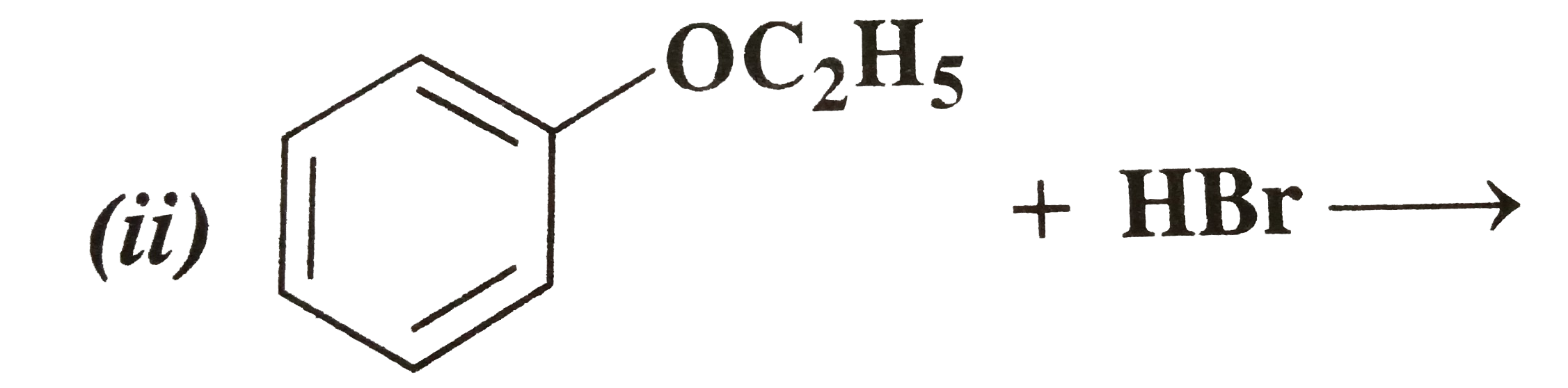
(iii)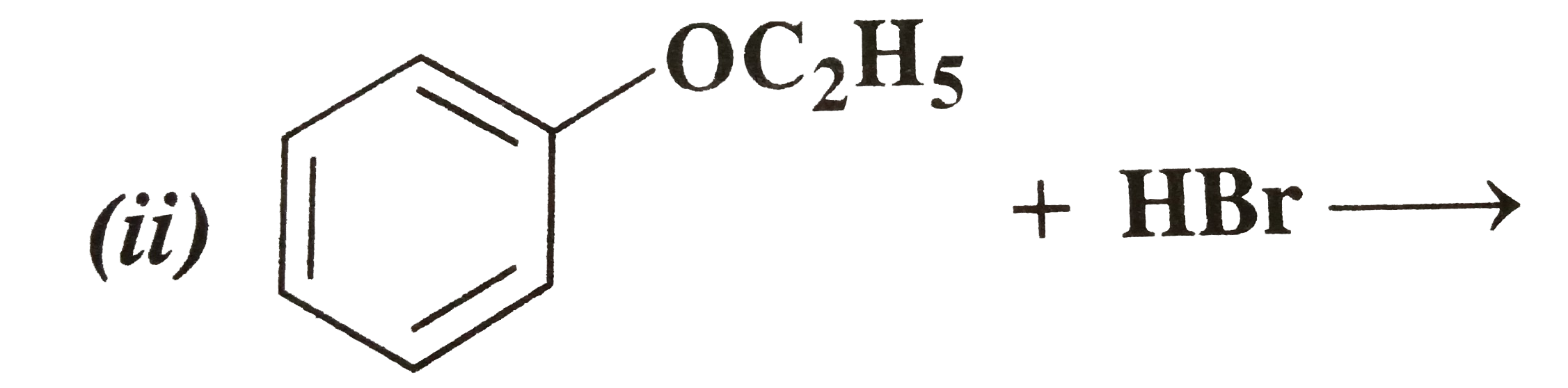
(iv) (CH3)3C−OC2H5+HI→
View SolutionThe major product of the following reaction is:
CH3−CH=CH2+HBr(C6H5CO)2O2 peroxide−−−−−−−−−−−−−−−→
View SolutionProduct of the following reaction is
CH3−Cl+C2H5−COOAg→AH3O+−−−→B
View SolutionFind out X in the following reaction
X+CH3OHconc.H2SO4−−−−−−−→C2H5COOCH3+H2O
View SolutionWhat will be the product of the following reaction?
CH3−CH=CH2+HBr(C6H5CO)2O2−−−−−−−−−→Product
View Solution