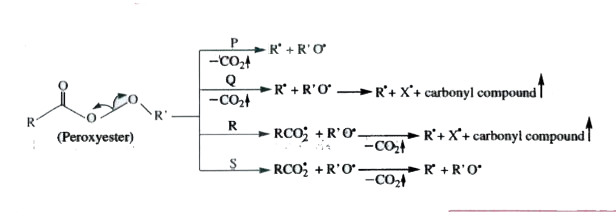
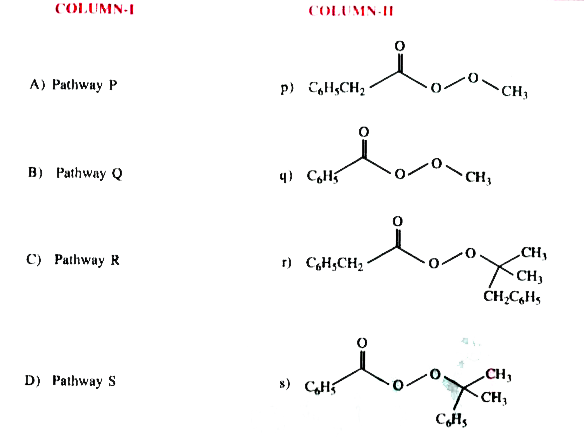
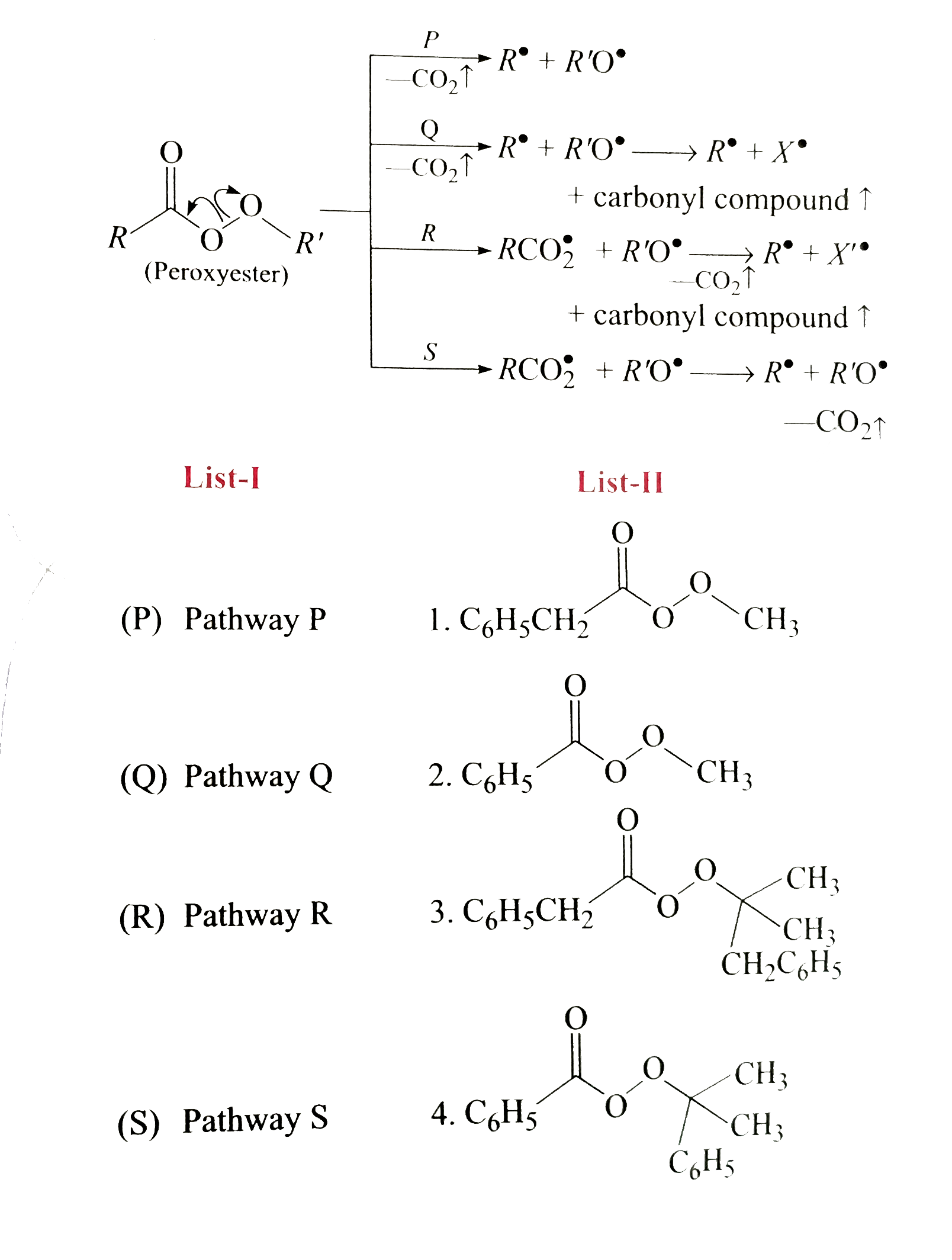
Different possible thermal decomposition pathwasy for peroxyesters are shown below. Match each pathway from List-I with an appropriate structure from List-II and select the correct answer using the code given below the lists
The correct Answer is:A, B, C, D
A→r,B→s,C→q,D⊤
Four pathways for reactions are given.
 .
.
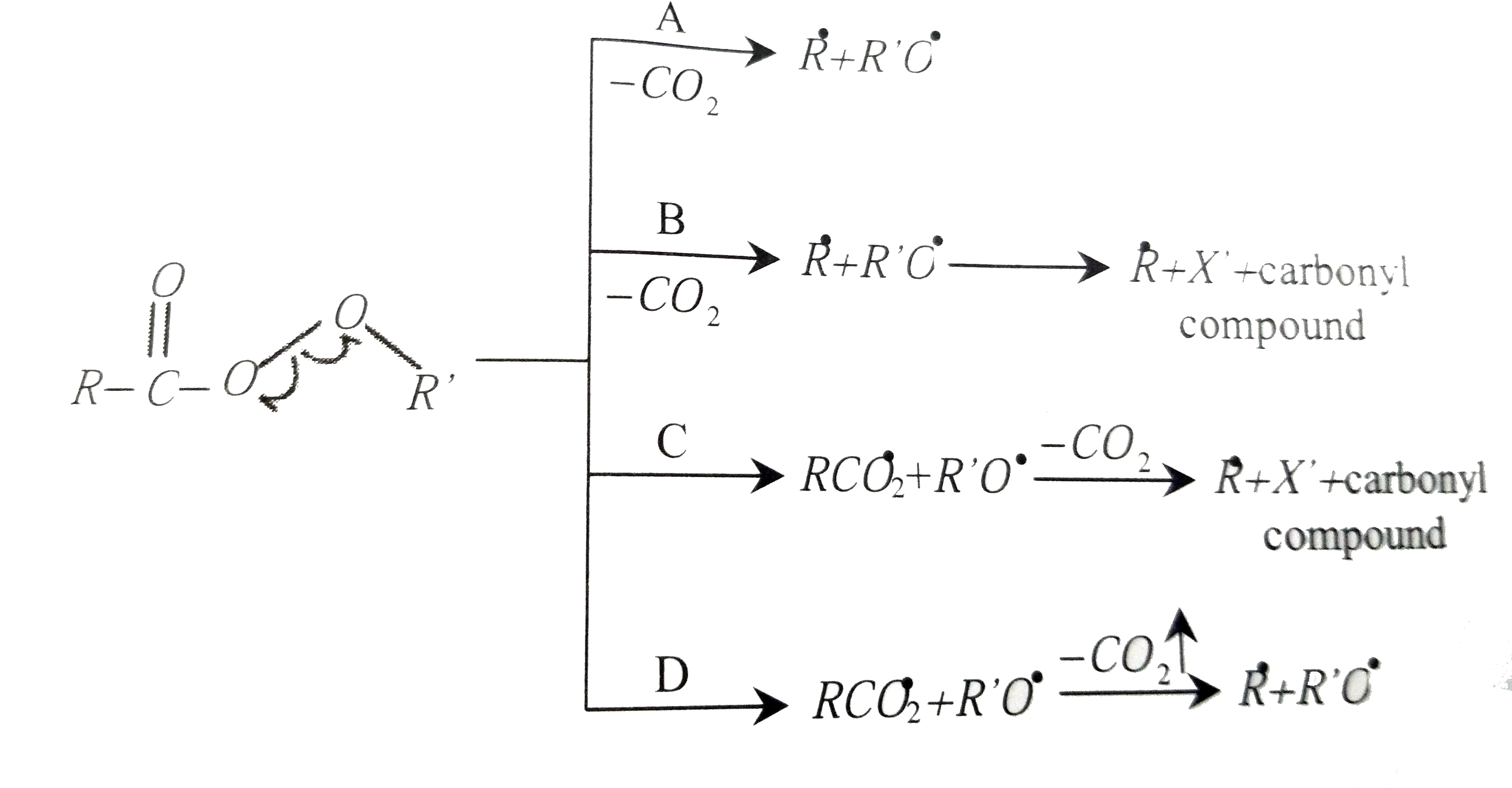
Note that first homolytic fission is expected to give R−O∣∣C−O∗
+R'O∗. This is usually unstable (remember kolbe's electrolyses)
and decomposes to R∗+CO2. But ph−O∣∣C−O∗ is stable, since it is a difficult bond to break. hence in (p) and (r), which have Ph−CH2−O∣∣C−O−O−R groups, Ph−CH∗2. will be formed (∵benzyl radical is stable) so (A) or (B) are possible
pathways for them. for Ph−O∣∣C−O−O−R (q and s), (C) and (D) pathways will be more likely.
∴1: A or B
3: C or D
2: A or B
4: C or D
Now consider R'O∗. if it is CH3O∗, it cannot splite.
But 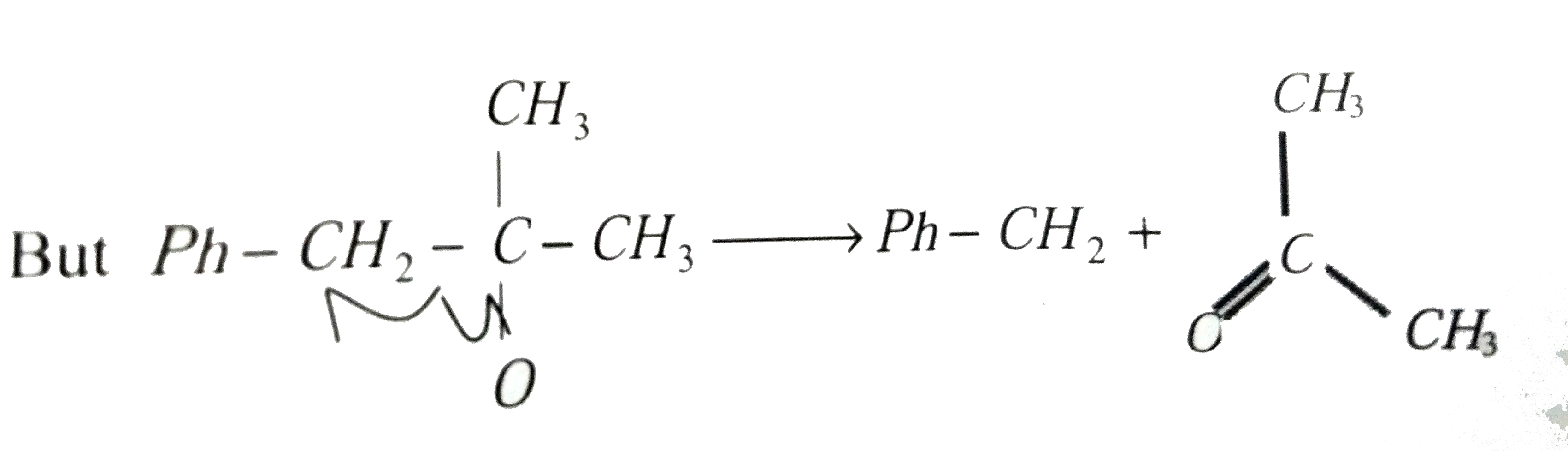
Can occurr. Hence carbonyl compound will be formed with r and s, but not with p and q.
∴1: A or D 3: B or C
2: A or D 4: B or C
Similar Questions
Different possible thermal decomposition pathways for peroxy esters are shown below. Match each pathway from list I with an appropriate structure from list II and select the correct answer using the code given below the list.
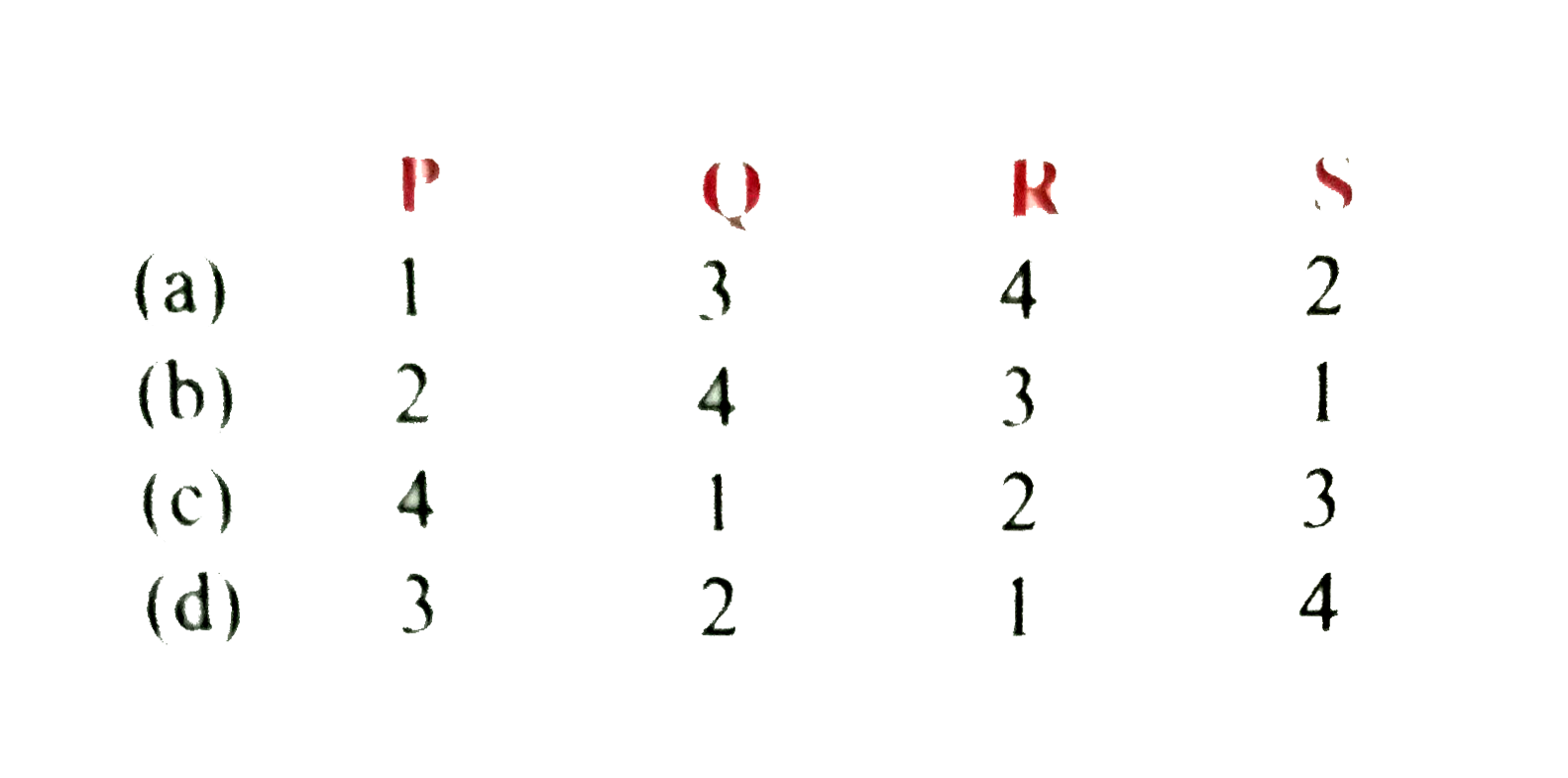
View SolutionMatch List-I with List-II and select the correct answer using the code given below the lists

View Solution
Knowledge Check
Match List-I with List-II and select the correct answer using the codes given below the lists :
 AABCD1234BABCD4321CABCD1321DABCD4231
AABCD1234BABCD4321CABCD1321DABCD4231Match List-I with List-II and select the correct answer using the code given below the Lists
AA-2,B-1,C-4,D-3BA-1,B-2,C-3,D-4CA-4,B-3,C-1,D-2DA-2,B-1,C-3,D-4Match List-I with List -II and select the correct answer using the code given below the Lists.
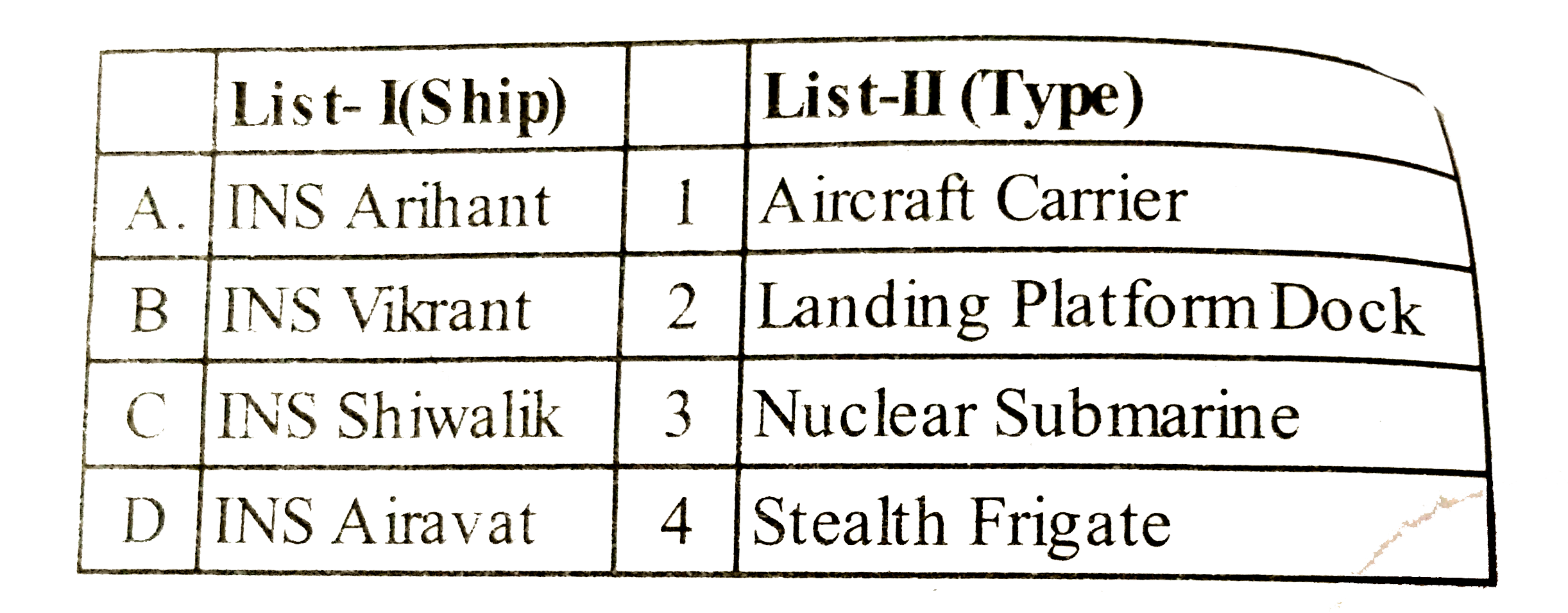 AA-3,B-4,C-1,D-2BA-3,B-1,C-4,D-2CA-2,B-1,C-4,D-3DA-2,B-4,C-1,D-3
AA-3,B-4,C-1,D-2BA-3,B-1,C-4,D-2CA-2,B-1,C-4,D-3DA-2,B-4,C-1,D-3
Match list I with List II and select the correct answer using the code given below the lists

View SolutionMatch List I with List II and select the correct answer using the code given below the lists

View SolutionMatch list-I with list-II and select the correct answer using the code given below the lists

View SolutionMatch list I with List II and select the correct answer using the codes given below the lists.

View SolutionMatch List I with List II and select the correct answer using the codes given below the lists:
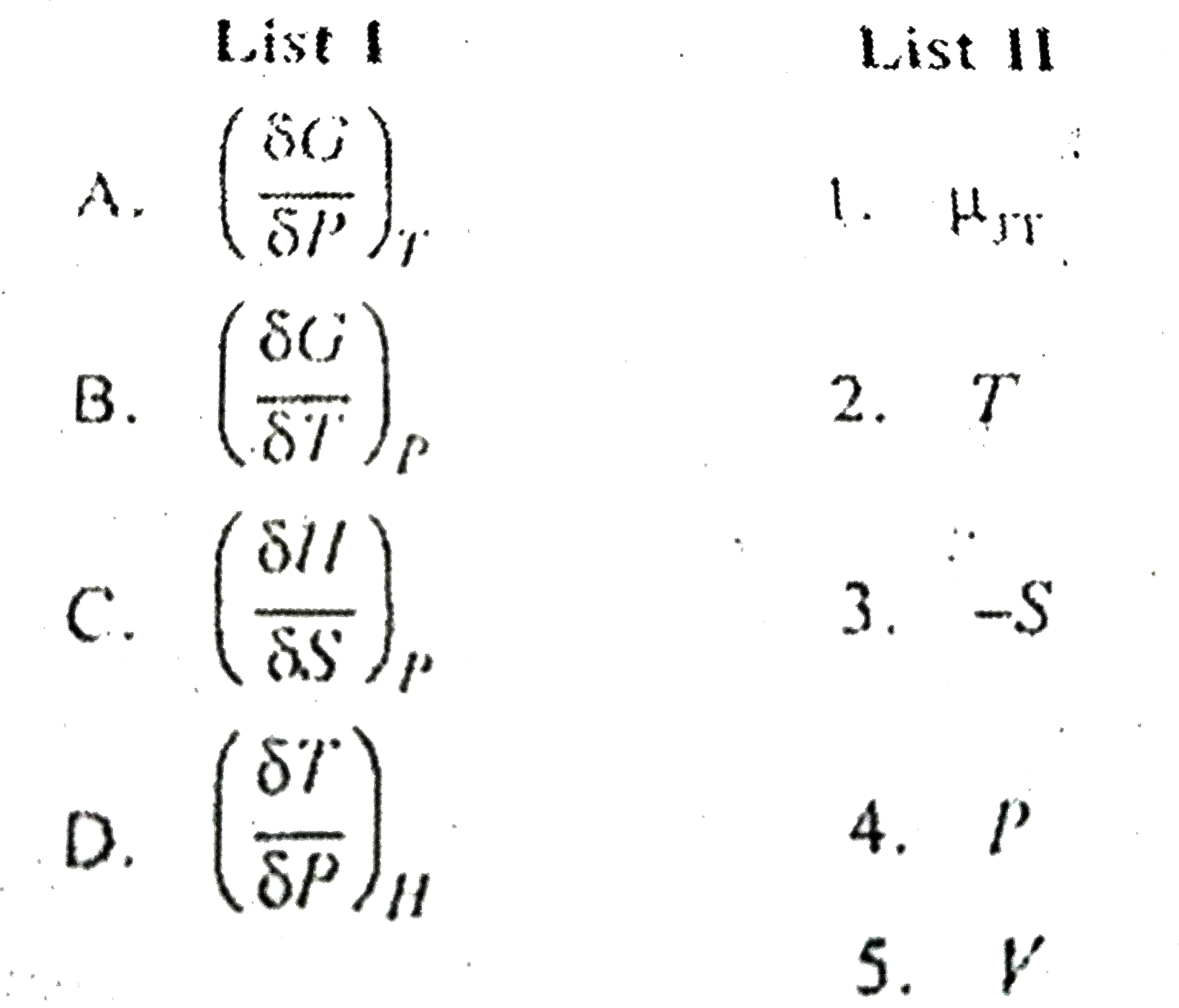
View Solution
