Match the extraction processes listed in Column I with metals listed in Column II.
Column IColumn IIA.Self reductionp.LeadB.Carbon reductionq.SilverC.Complex formation and displacement by metal r.CopperD. Decomposition of iodides.Boron
The correct Answer is:A, B, C, D
"Selt reduction" " " [p−Leadr−Copper]
In head metal
{2PbS+3O2→2PbO+2SO2(Slef reduction)2PO+PbS→3Pb+SO2
In copper metal
2Cu2S+3O2→2Cu2O+2SO2
Cu2S+2Cu2→6Cu2+2SO2 (Slef reduction)
(B) Carbon reduction p- Lead
In lead metal
PbO+C→Pb+CO
PbO+CO→Pb+CO2
Carbon reduction process is used for the reduction of oxides of electropositive metals like , Fe, Pb etc. This reduction is carried out with coal or coke on strongly heating.
(C) Comlex formation and displacement by meta.
In silver metal
Average (Ag2S) mineral of Ag is treated iwth 0.7%NaCN solution in presence of air, therefore, firstly soldium argentocyanide compled is formed which on heating with zinc meta, therefore, by displacement silver is isolated
Ag2S+4NaCN→2Na[Ag(CN)2]+NaS
2Na[Ag(CN)2]+Zn→Na2[Zn(CN)4]soluble+2Agppt⏐⏐
⏐↓
(D) Decomposition of iodide _______ (S) Boron By decomposition of iodide boron metal is isolated.
2BI3Wm heat−−−−→2B+3I2
Similar Questions
Match the extraction processes listed in Column I with metals listed in Column II.
Column IColumn IIA.Self reductionp.LeadB.Carbon reductionq.SilverC.Complex formation and displacement by metal r.CopperD. Decomposition of iodides.Boron
View SolutionMatch the reaction products listed in column-I with the particulars listed in column-II
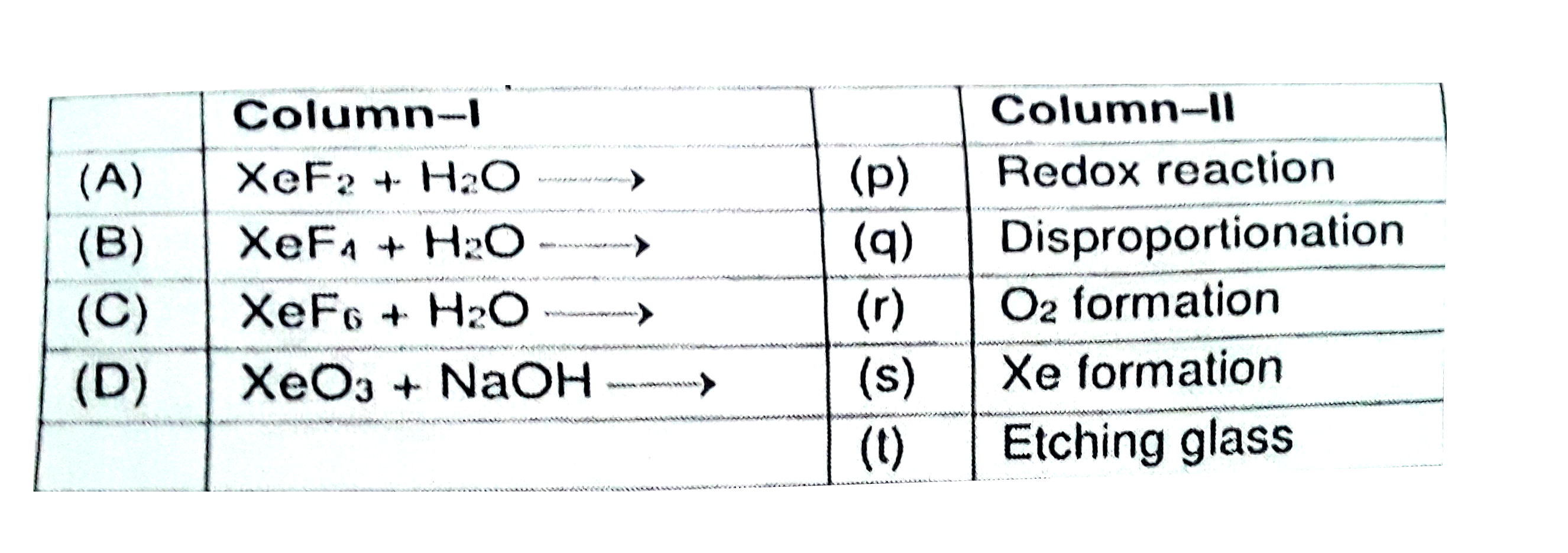
View Solution
Knowledge Check
Match the reaction listed in coloumn-I with characteristic(s) listed in column-II
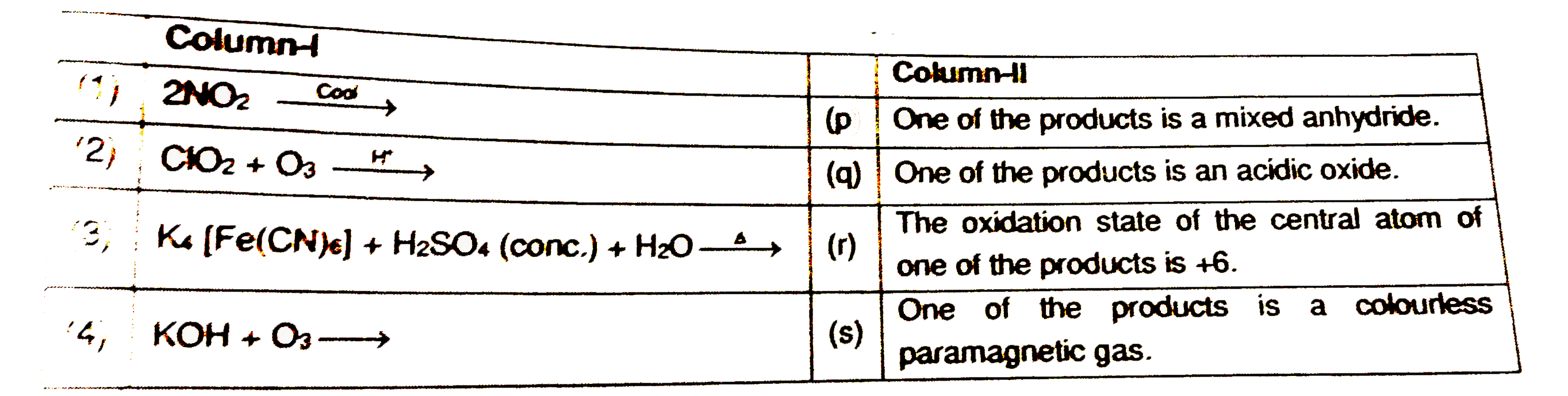 A1−p,q,2−p,q,s,3−r,4−sB1−p,q,2−p,q,r,s,3−r,4−sC1−p,2−p,q,r,s,3−r,4−sD1−p,q,2−q,r,s,3−s,4−r
A1−p,q,2−p,q,s,3−r,4−sB1−p,q,2−p,q,r,s,3−r,4−sC1−p,2−p,q,r,s,3−r,4−sD1−p,q,2−q,r,s,3−s,4−r
Match the extraction process in column I with the metal in column II.
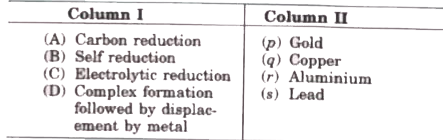
View SolutionMatch the reactions listed in column-I with the products listed in column-II
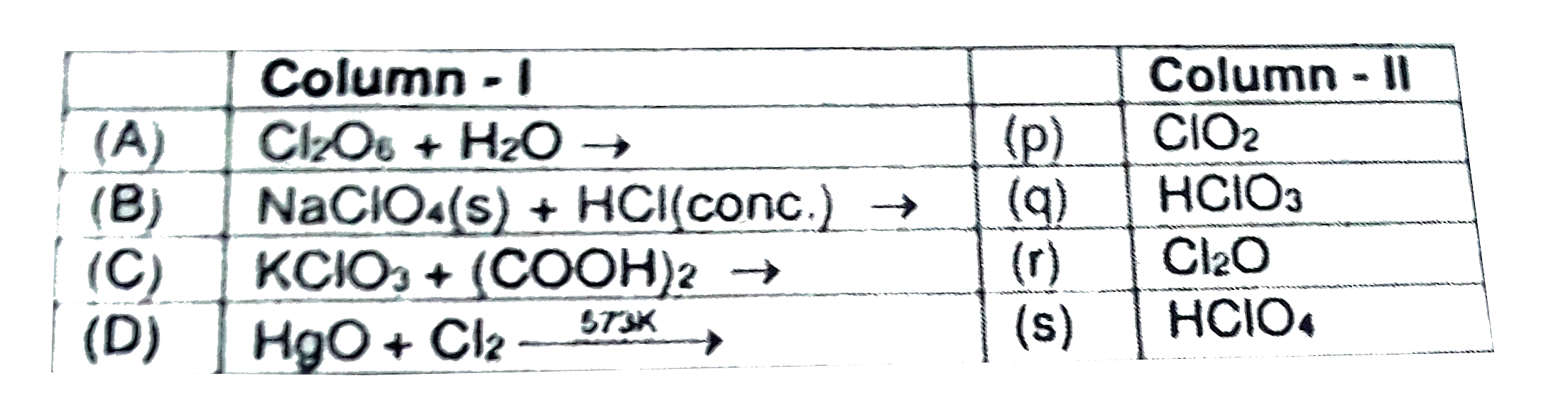
View SolutionMatch the reactions listed in column-I with the products listed in column-II
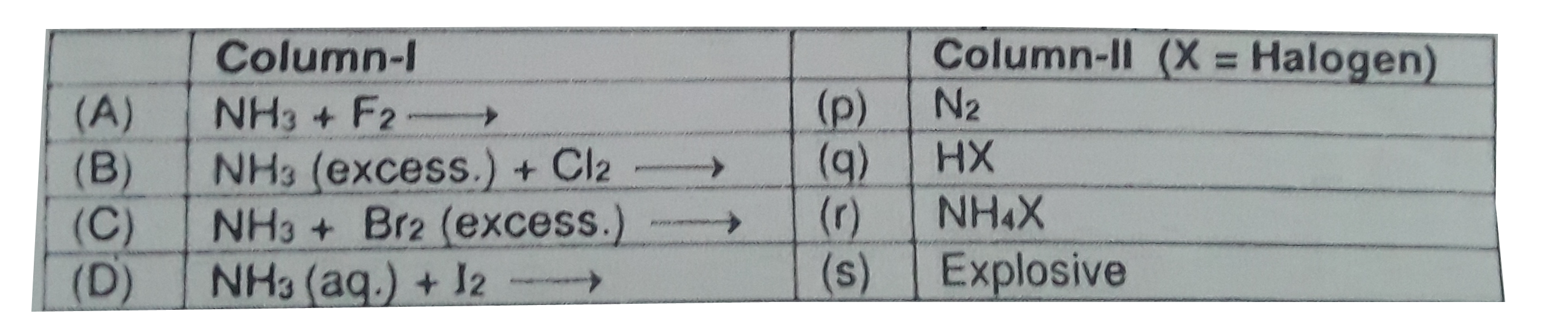
View SolutionMatch the complexes listed in column-I with characteristic(s) listed in column-II.
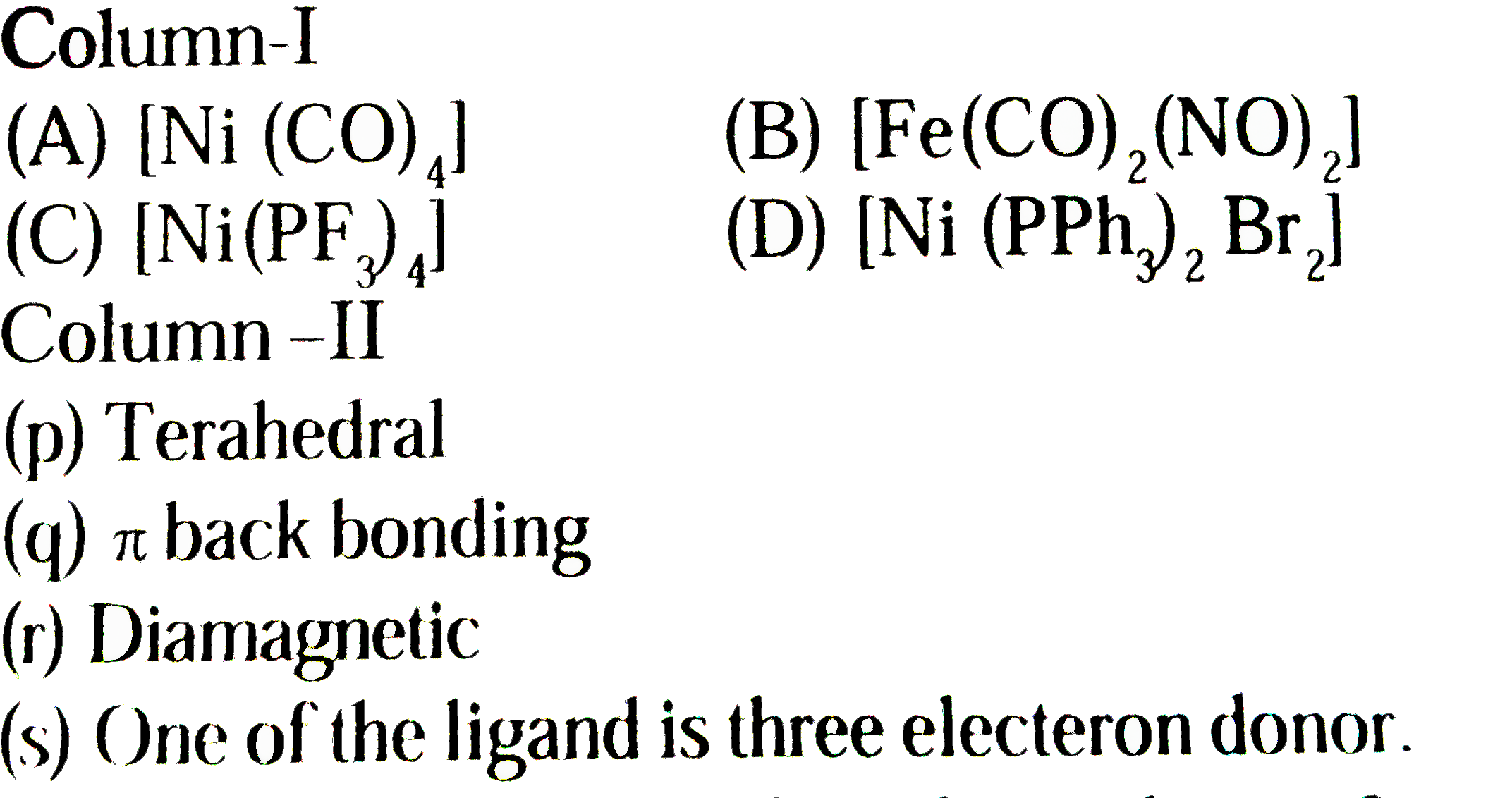
View SolutionMatch the compound listed in Column I with characteristic listed in Column II

View SolutionMatch the properties given in Column I with the metals given in Column II

View SolutionMatch the properties given in Column I with the metals given in Column II.
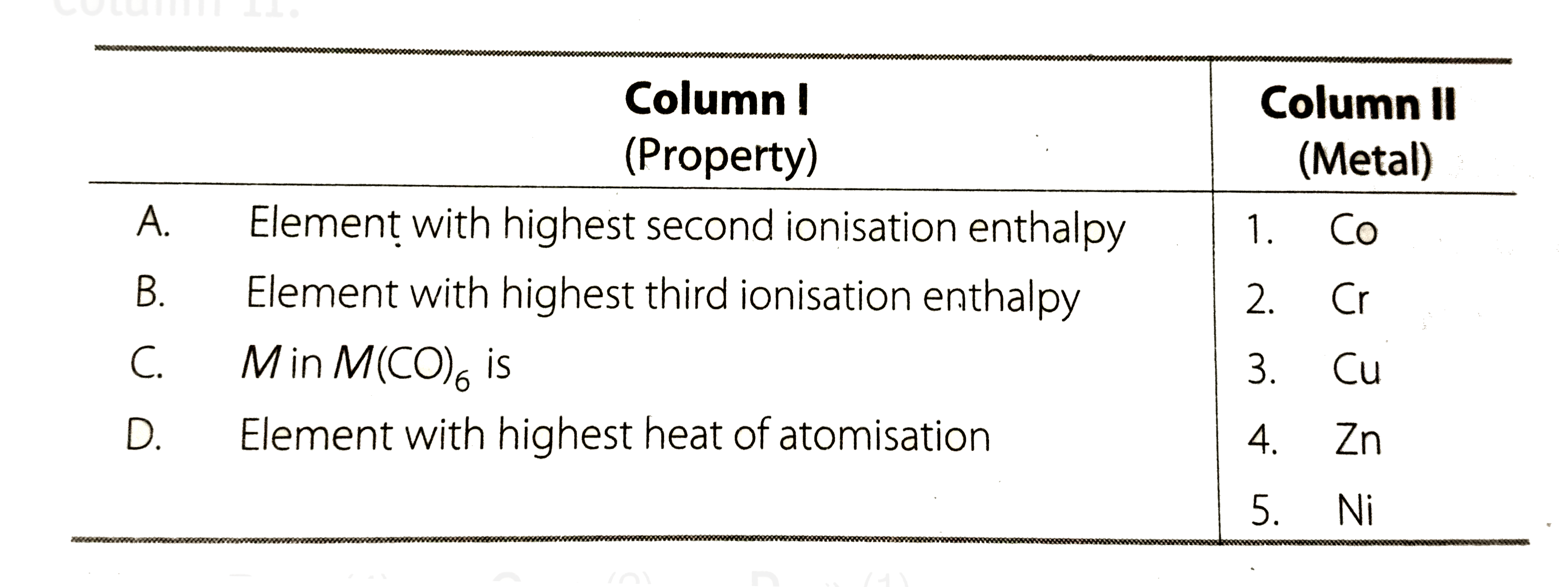
View Solution
